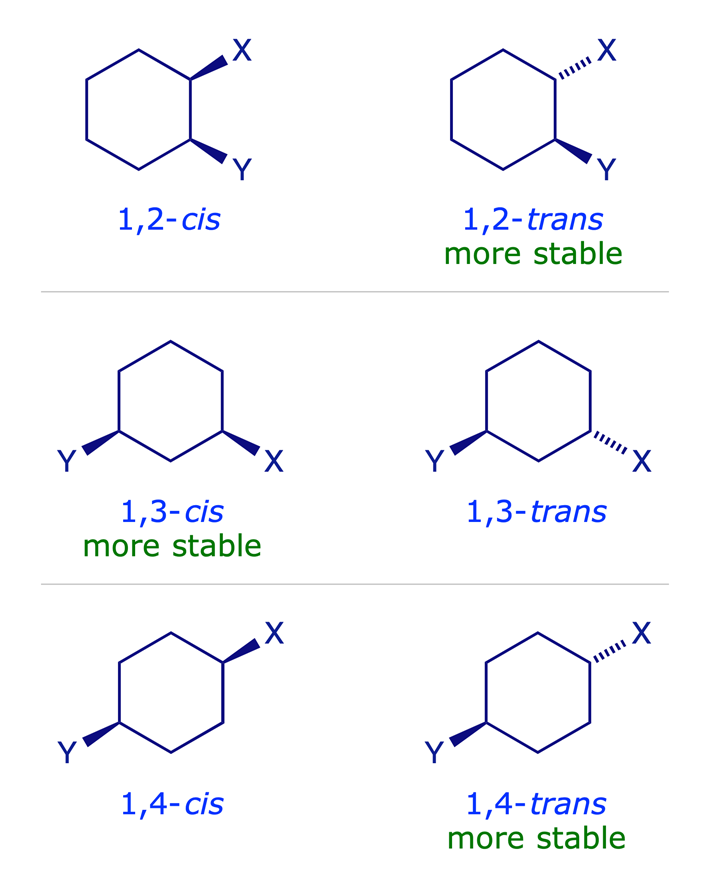
Disubstituted cyclohexanes
When a single substituent is present on a cyclohexane ring, it tends to adopt an equatorial orientation so as to minimise 1,3-diaxial steric repulsions (cf. methylcyclohexane). In disubstituted systems the conformational preference depends on the substitution pattern. The lower energy arrangement has the larger of the two groups in an equatorial position, so for X = Y in the graphic below, the all-equatorial isomers are the more stable of each pair.
Use the links below to explore the structures of the dimethylcyclohexanes with JSmol.
×
![]()
Go to methylcyclohexane
Go to 1,2-dimethylcyclohexane
Go to 1,3-dimethylcyclohexane
Go to 1,4-dimethylcyclohexane
