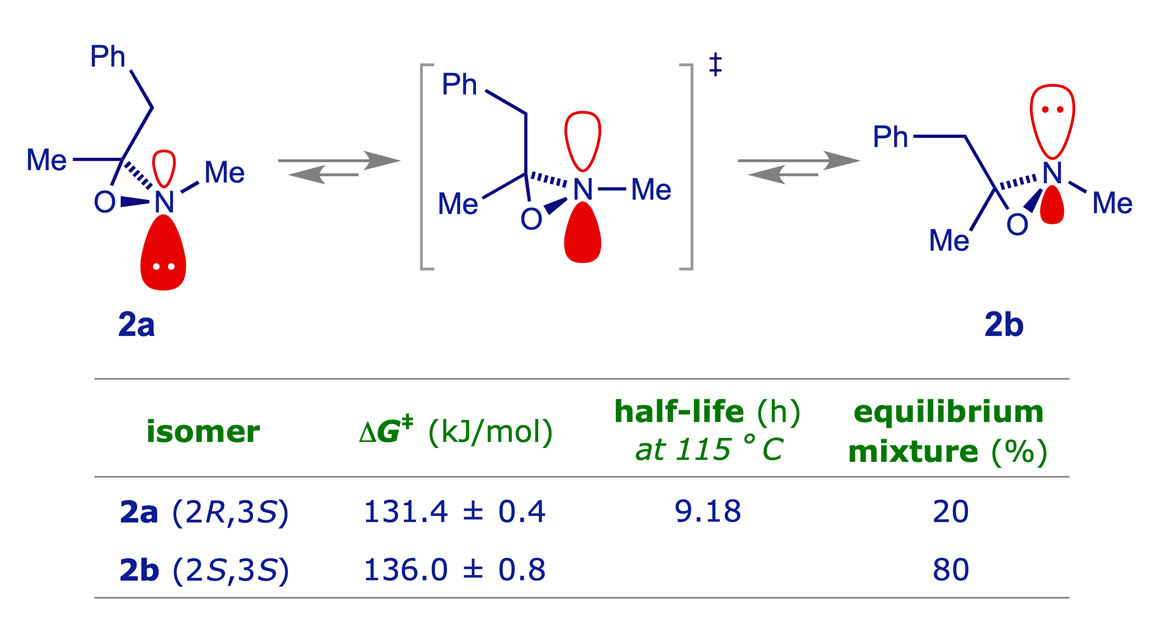
Oxaziridines 2a and 2b
The energy barrier for inversion of a tetrahedral (sp3) nitrogen atom is influenced by various structural features.
- The barrier is lowered if the N atom bears a pi-acceptor group. This stabilises the transition state structure, in which the N atom is planarised with the lone-pair in a pure 2p orbital, by delocalisation.
- The barrier is raised if the N atom bears an electronegative substituent X. This is due to the inductive withdrawal of electron density through the N–X sigma bond, which deshields the N nucleus so that electrons with s character, in particular, are more tightly bound. This widens the energy gap between the lone-pair orbitals of N in its pyramidal (sp3) and planarised (2p) states.
- The barrier is raised if the N atom is part of a three-membered ring. This gives rise to high strain in the transition state structure, where N is planarised but the bond angles are necessarily constrained to about 60°.
The diastereoisomeric oxaziridines 2a and 2b are configurationally stable at room temperature and separable by chromatography. They equilibrate on heating at 115 °C, with 2b predominant by about 4:1. Oxaziridines tend to be resistant to inversion at N due to both the strain of the three-membered ring and the inductive (sigma-attractor) effect of the endocyclic O atom.
Proton-resonance studies of the inversion at tervalent nitrogen atoms, III. Separation of the invertomers of 2,3-dimethyl-3-benzyloxaziridine; A. Mannschreck, J. Linss and W. Seitz, Justus Liebigs Ann. Chem., 1969, 727, 224–227 (DOI: https://doi.org/10.1002/jlac.19697270126).
