1-Chloro-2,2-diphenylaziridine
The energy barrier for inversion of a tetrahedral (sp3) nitrogen atom is influenced by various structural features.
- The barrier is lowered if the N atom bears a pi-acceptor group. This stabilises the transition state structure, in which the N atom is planarised with the lone-pair in a pure 2p orbital, by delocalisation.
- The barrier is raised if the N atom bears an electronegative substituent X. This is due to the inductive withdrawal of electron density through the N–X sigma bond, which deshields the N nucleus so that electrons with s character, in particular, are more tightly bound. This widens the energy gap between the lone-pair orbitals of N in its pyramidal (sp3) and planarised (2p) states.
- The barrier is raised if the N atom is part of a three-membered ring. This gives rise to high strain in the transition state structure, where N is planarised but the bond angles are necessarily constrained to about 60°.
In the case of the chloroaziridine 1, the pyramidal N atom bears an electronegative chlorine and is part of a strained three-membered ring. This combination gives rise to significant configurational stability at room temperature.

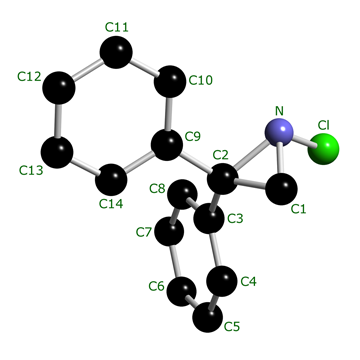
Absolute configuration of a chiral N-chloroaziridine with molecular asymmetry due solely to a trivalent nitrogen atom. X-Ray structure of (R)-(−)-1-chloro-2,2-diphenylaziridine; S. Brückner, A. Forni, I. Moretti and G. Torre, J. Chem. Soc., Chem. Commun., 1982, 1218–1219 (DOI: https://doi.org/10.1039/C39820001218).
Relationships between solid-state structures of enantiomers and the corresponding racemic compounds in small ring derivatives. Comparison of crystal structures and solid-state properties of (R)-(–)- and racemic 1-chloro-2,2-diphenylaziridine. Solvent effect on the racemization of (R)-(–)-1-chloro-2,2-diphenylaziridine; A. Forni, I. Moretti, G. Torre, S. Brückner, L. Malpezzi and G. Di Silvestro, J. Chem. Soc., Perkin Trans. 2, 1984, 791–797 (DOI: https://doi.org/10.1039/P29840000791).
