Stereospecific reactions
The SN2 reaction
Consider a reaction being carried out on starting materials that are stereoisomeric, e.g. a pair of enantiomers. If the reaction gives a product from one of the starting materials that is a stereoisomer of the product from the other, then the reaction is deemed to be stereospecific. A reaction is stereospecific by virtue of a strict reaction mechanism, and you have already encountered two examples of these: One of these is the E2 elimination reaction, in which a haloalkane is transformed into an alkene by treatment with base. Such reactions always proceed through a transition state in which the five reacting centres assume an antiperiplanar arrangement. The other is SN2 substitution, which can be relied upon to involve stereochemical inversion of the reacting centre.
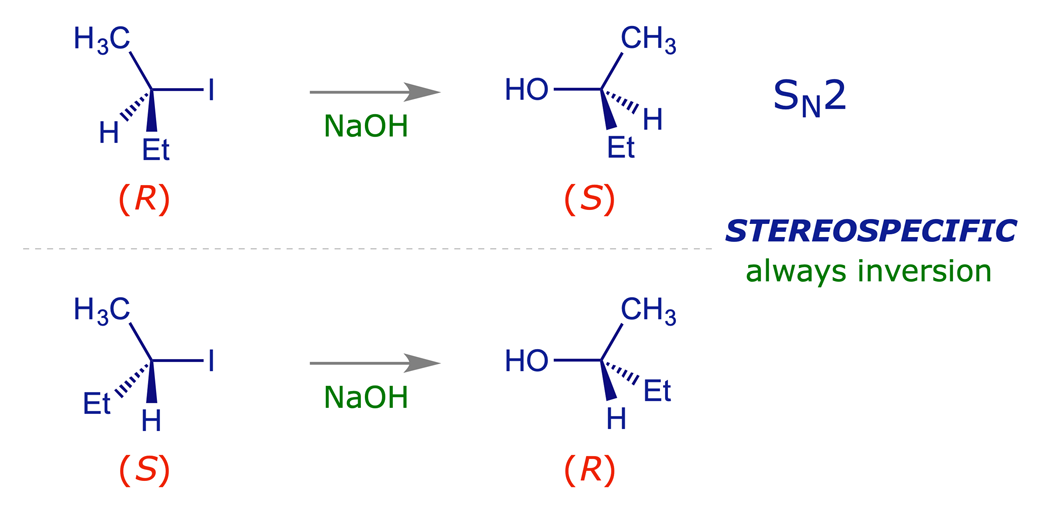
The interactive model below illustrates the SN2 reaction between bromomethane and chloride anion. The substitution always proceeds with inversion at the reacting carbon atom and this is what defines it as a stereospecific process.
