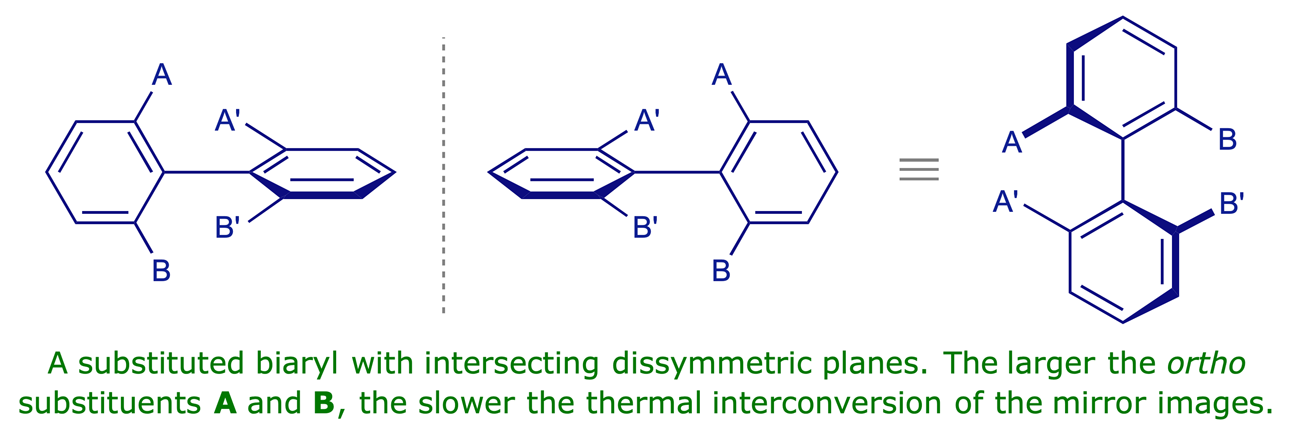
The stability of atropisomers
Atropisomers ('non-turning isomers') are enantiomeric conformations that are separable because rotation around a single bond is sterically hindered. This phenomenon is encountered in substituted biaryls. As in the case of allenes, the crucial geometric feature in such compounds is intersecting dissymmetric planes, neither of which is a plane of symmetry.
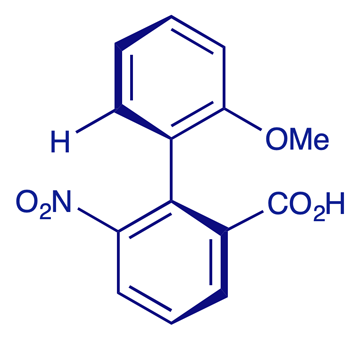
2'-Methoxy-6-nitrobiphenyl-2-carboxylic acid, shown below, was studied by Adams and Li during the 1930s. They were able to isolate a single enantiomer of this acid but it quickly racemised, losing its optical activity with a half-life of 12 min in EtOH at 24°C.
An interactive model of this acid is shown below. In this compound, rotation about the Ar–Ar bond is inhibited but not prevented by the substituents in the positions next to the inter-aryl bond — one of the substituents is hydrogen (i.e. small), so the (R)- and (S)-configurations of this biaryl can interconvert at room temperature. To visualise the limited 'free rotation'of the Ar–Ar single bond in this compound, try the following sequence of settings:
reset → Atoms 100% → play reversibly → rotate x 90°
Stereochemistry of diphenyls. XL.1 The effect of temperature and solvent on the rate of racemization of 2-nitro-6-carboxy-2' -alkoxydiphenyls; C. C. Li and R. Adams, J. Am. Chem. Soc., 1935, 57, 1565–1569 (DOI: 10.1021/jo00950a020).
