Cyclohexane
Cyclohexane is not planar but puckered in a 3-D conformation that relieves strain. Its most stable arrangement is referred to as the chair conformation. The different chair conformations of cyclohexane can interconvert or 'flip' very easily: the activation barrier is low — about 45 kJ/mol.
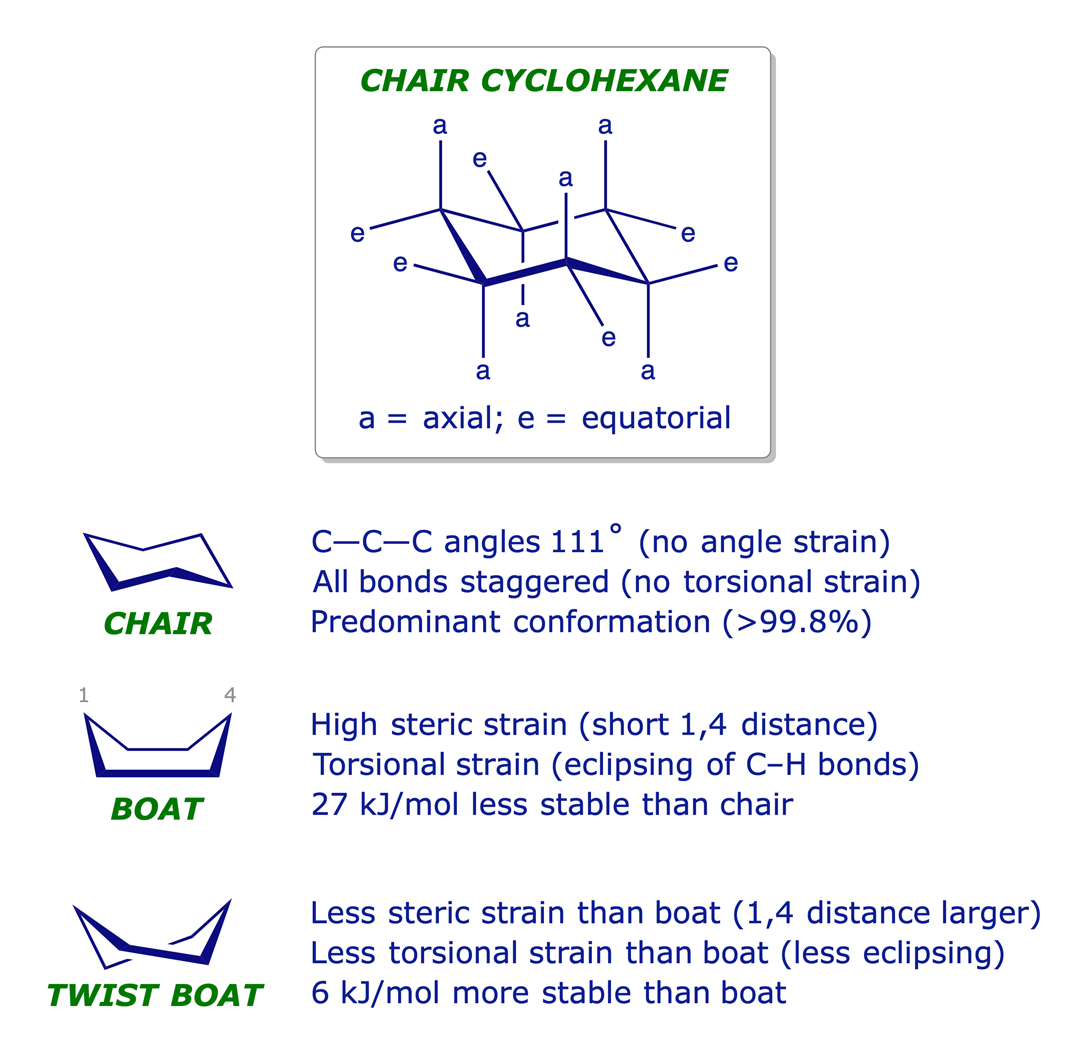
×
![]()
Use the interactive model below to explore the conformations and ring flipping of cyclohexane (for the animation, six of the hydrogen atoms can be coloured green so that you can more easily distinguish axial atoms from equatorial atoms).
Go to conformations of butane
Go to methylcyclohexane
