P-Chiral phosphines
While ammonia and phosphine are both pyramidal, the H–P–H bond angles of PH3 are close to 90° and the dipole moment is only 0.58 D (compared to 107° and 1.47 D respectively for ammonia). These values indicate that the P–H bonds of phosphine involve mainly the p-orbitals of phosphorus, with very little contribution from the 3s orbital. As a result the P lone pair shows more s-character, and is quite different to the N lone pair in ammonia. Phosphines are less nucleophilic and less basic than analogous amines, while the inversion barrier in phosphine is about 130 kJ mol−1 compared to only 24 kJ mol−1 in ammonia. The structures of NH3 and PH3 shown here were generated by quantum chemical calculation (Møller-Plesset RMP2, 6-311+G**).
Trisubstituted phosphine enantiomers do not readily racemise and the inversion barrier is relatively insensitive to the substituents. The thermal racemisation of enantiomerically pure methylphenylpropylphosphine was studied by Horner and Winkler in the 1960s. The kinetics of the process indicated an inversion barrier (Ea) in the range 120-130 kJ mol−1, with some solvent dependency.
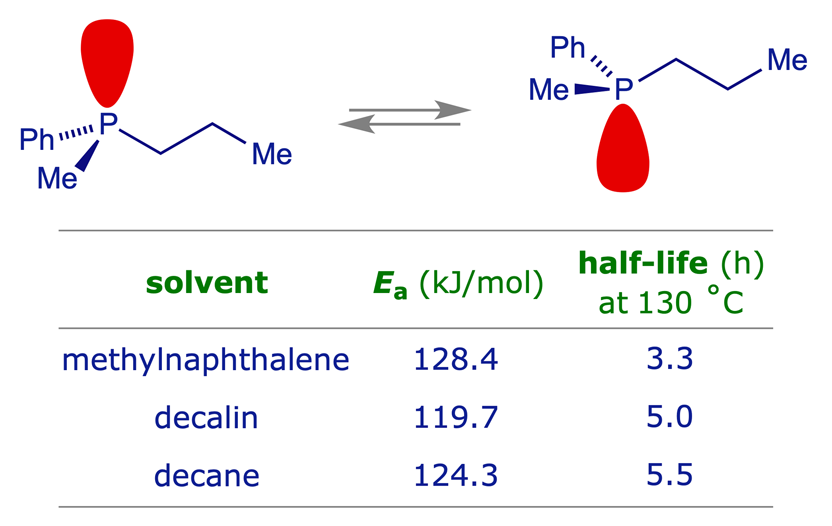
For details of the study on methylphenylpropylphosphine:
L. Horner and H. Winkler, Tetrahedron Lett., 1964, 5, 461–462 (https://doi.org/10.1016/S0040-4039(00)90361-7).
For information on other P-stereogenic phosphines:
K. V. L. Crépy and T. Imamoto, Top. Curr. Chem., 2003, 229, 1–40 (https://doi.org/10.1007/b11148).
