6 Separation of enantiomers
6.1 The separation of enantiomers
Enantiomers have identical physical properties, and if we want to distinguish between them or separate them, the enantiomeric relationship must be converted into a diastereoisomeric one. This requires another source of chirality, e.g. an enantiomerically pure chiral reagent, and a procedure that can be applied on a preparative scale. The overall strategy for this process, called resolution, is illustrated in the scheme below.
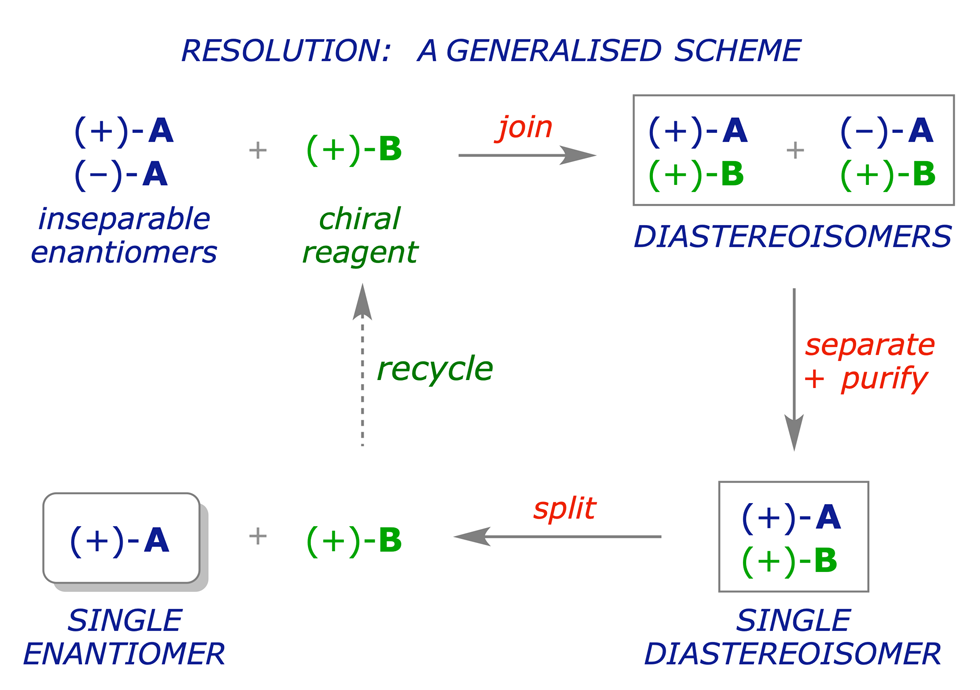
While the principles underlying the above sequence are easy to understand, the resolution of enantiomers on a preparative scale can present a series of practical challenges. The join, separate and split processes must not only be efficient but also chemically suitable. In principle, diastereoisomeric intermediates resulting from any type of interaction between A and B might be useful, including:
- Ionic bonding
- Covalent bonding
- Weak coordinate bonding (hydrogen bonds, dipole-dipole interactions, pi/pi interactions, etc.)
6.2 Resolution via salt formation (ionic bonding)
The simplest examples of resolution involve the neutralisation of acids or bases in order to convert them into a separable pair of diastereoisomeric salts. This is the easiest method of resolution and has often been used for the resolution of racemic acids (using an amine as the resolving agent) and bases (using an acid as the resolving agent). This type of resolution is generally efficient for the following reasons:
- Diastereoisomeric salts can often be separated by crystallisation. Recrystallisation gives high diastereoisomeric purity and the process is practical on a large scale.
- The pure enantiomers can easily be separated from the resolving agent at the end of the process by simple solvent (acid/base) extraction, which is again practical on a large scale.
The following examples illustrate some of the practical aspects of resolution via salt formation.
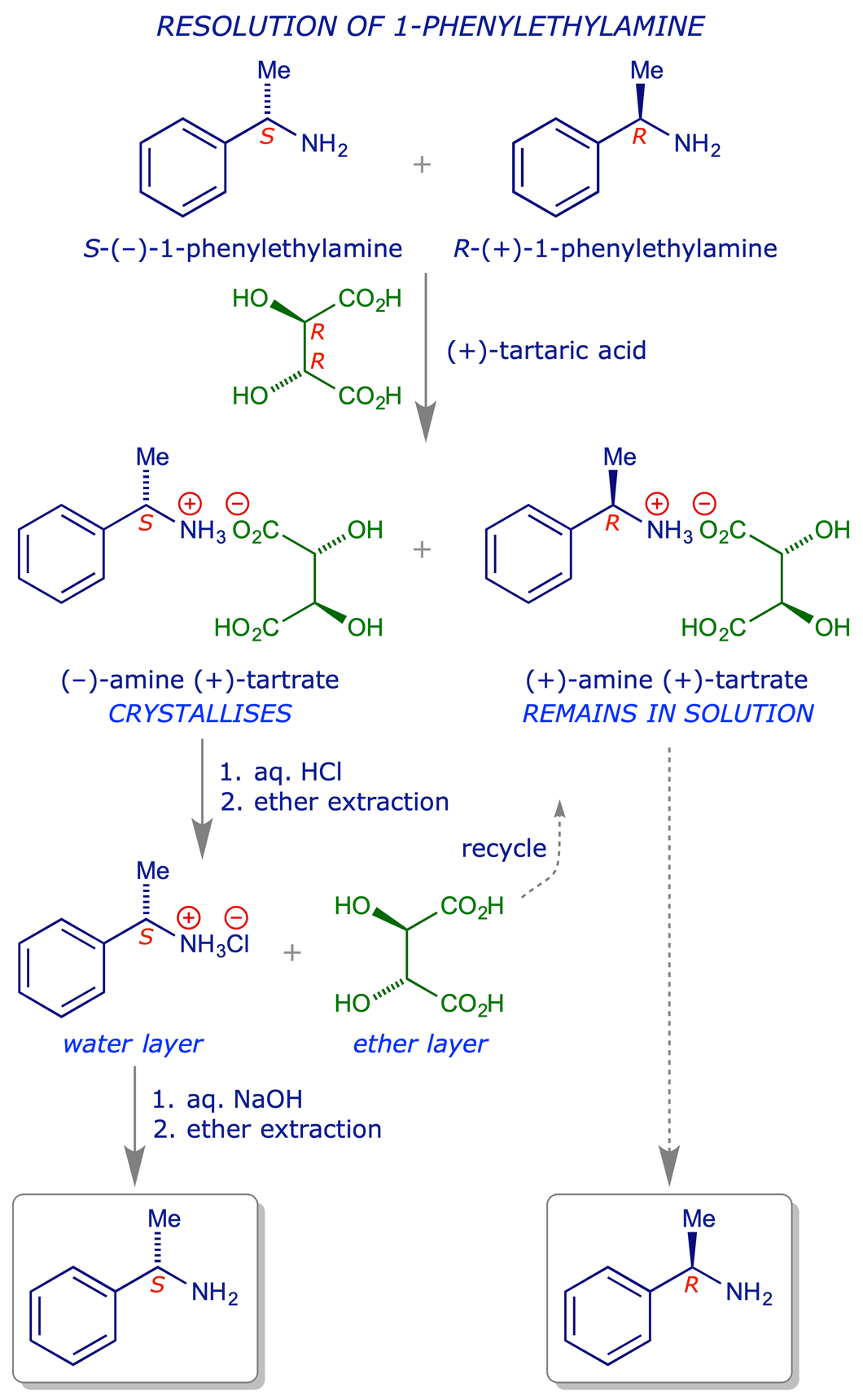
6.2.1 Resolution of 1-phenylethylamine (α-methylbenzylamine)
The title amine has long been recognised as a versatile chiral reagent, both of the pure enantiomers being inexpensive and available in quantity. It can be resolved using the natural form of tartaric acid.
6.2.2 Resolution of 2-(6-methoxy-2-naphthyl)propanoic acid (Naproxen)
A series of simple 2-arylpropanoic acids, referred to as NSAIDs (non-steroidal anti-inflammatory drugs), have analgesic, antipyretic and anti-inflammatory properties which make them valuable for reducing pain, fever and inflammation. A well-known example is (S)-naproxen, which was first marketed by Syntex in 1976. The significance of this drug is reflected in its sales figures, which topped $1 billion for the first time in 1995.
The original manufacture of naproxen included a classical resolution using the naturally-occurring amine, cinchonidine, to form diastereoisomeric salts. It was later found that various N-alkyl-D-glucamines, which are easy to make by reductive amination of D-glucose, are also effective resolving agents for naproxen. A sequence for the resolution of (S)-naproxen using N-octyl-D-glucamine is illustrated below.
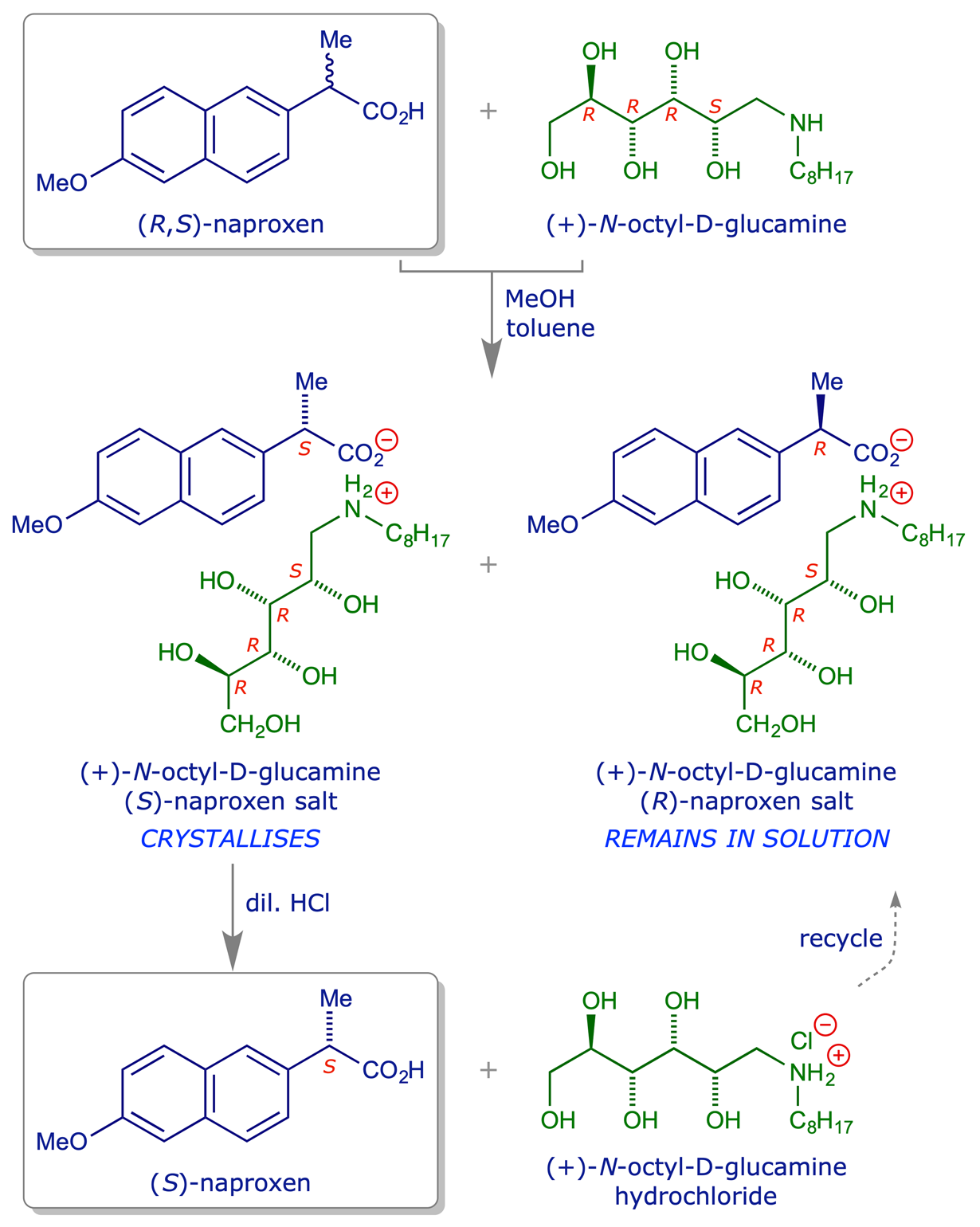
More recently the manufacture of naproxen was refined by the incorporation of a racemisation step, in which the unwanted (R)-naproxen is converted into (R,S)-naproxen and recycled through the resolution loop. Together with refinements to the amine recycling step, the resolution process has been refined to the point where the yield of (S)-naproxen from racemic material is over 95%, with an enantiomeric purity of 99%, and the recovery yield of the amine resolving agent is more than 98% per cycle.
For more on this topic, see also Twenty Years of Naproxen Technology: P. J. Harrington and E. Lodewijk, Org. Process Res. Dev., 1997, 1, 72–76.
6.3 Resolution via covalent bond formation
In principle any functional group of a racemic compound can be reacted with a chiral reagent so as to generate diastereoisomeric products, which might then be separable. However, for an effective resolution it must be possible to reverse the reaction used for diastereoisomer formation. This imposes a limit on the number of reactions which are useful for resolution purposes, and the most common covalent reaction used is esterification. The chemistry is of course complementary — it can be used to resolve (i) chiral alcohols or (ii) chiral carboxylic acids.
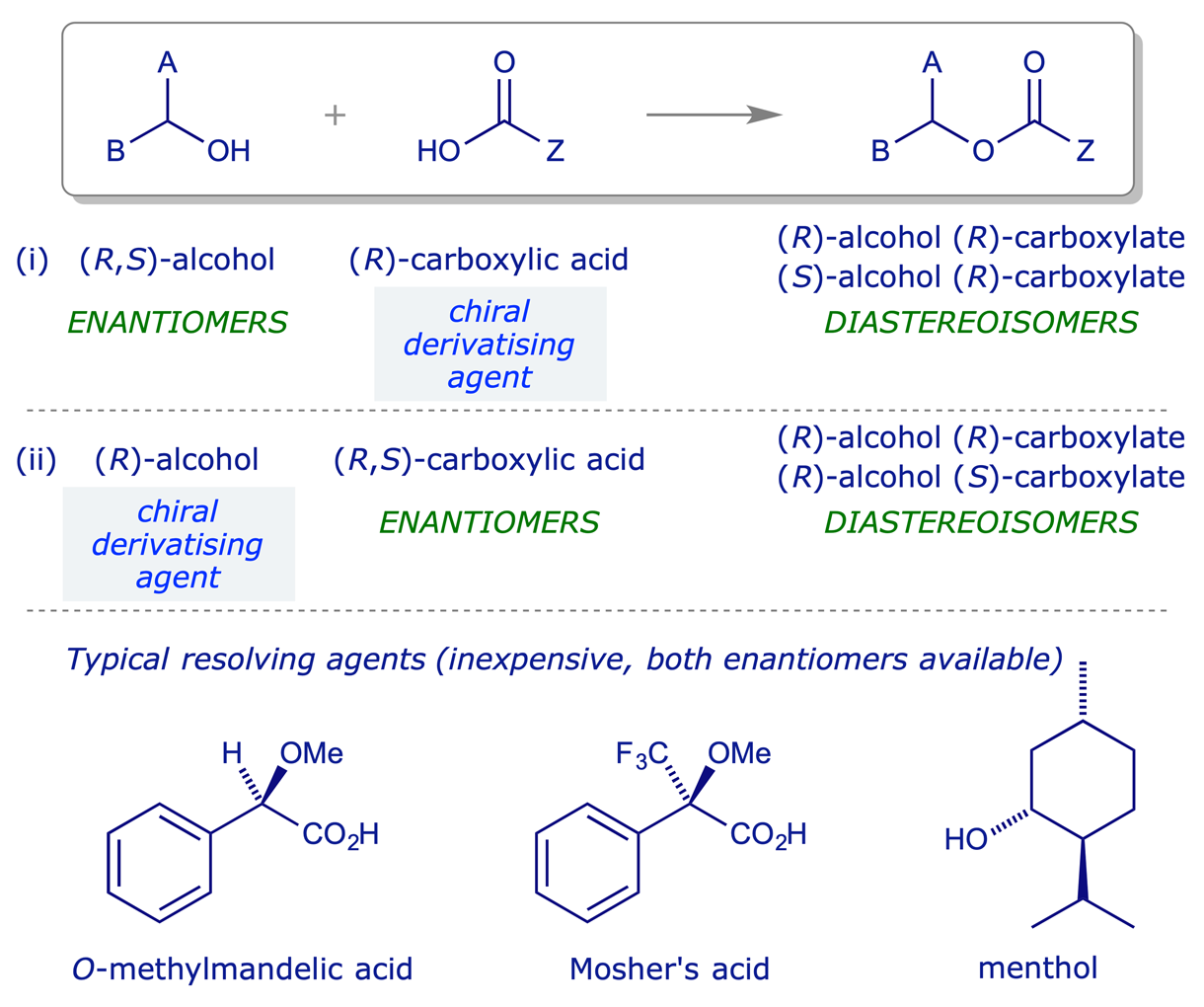
Example resolution:
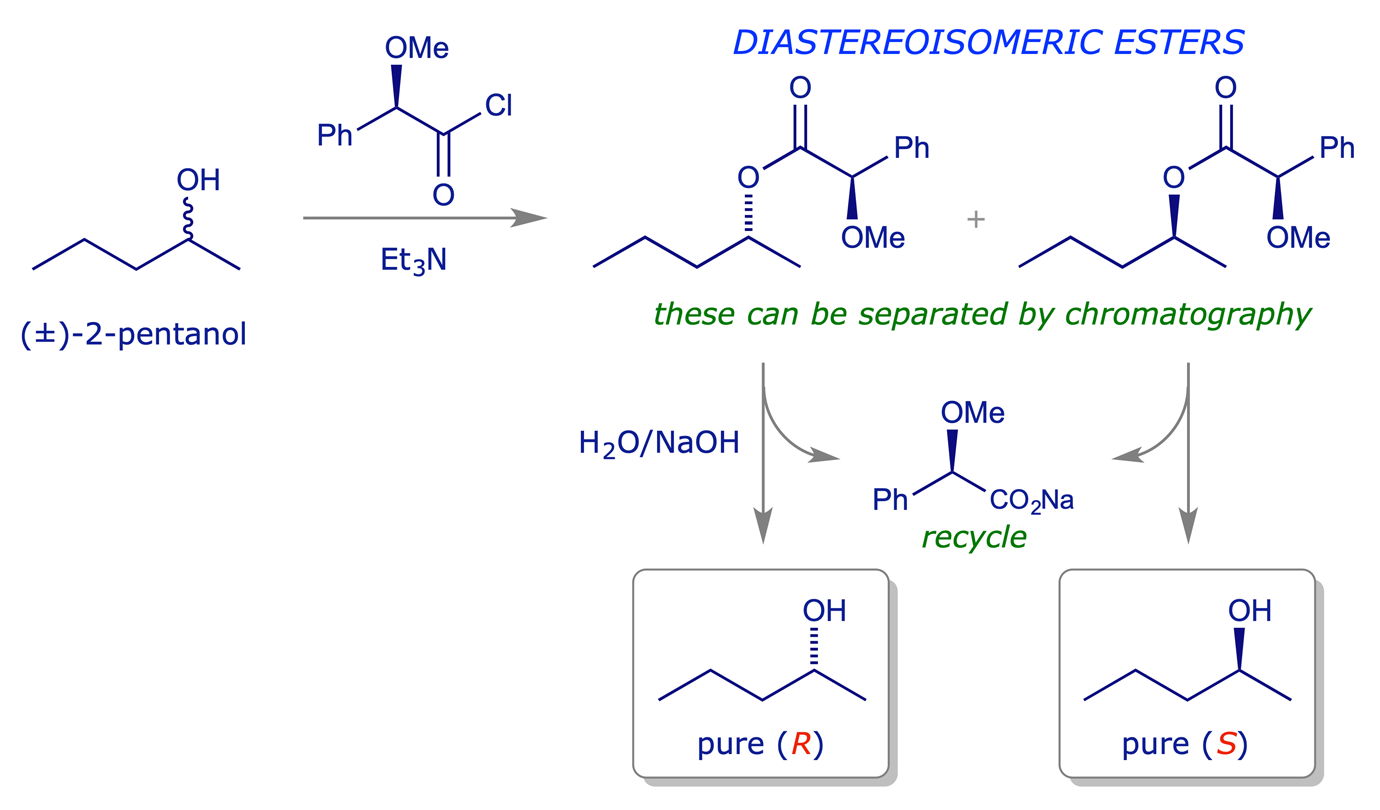
A drawback with this type of resolution is that the diastereoisomeric esters normally have to be separated by chromatography, which is impractical on a large scale. However, it is often easy to find a resolving agent for this type of process compared to one involving fractional crystallisation. Another advantage is that the diastereoisomeric products can often be separated to high isomeric purity, verifiable by NMR, HPLC etc. before the hydrolysis step. It is thus a good method for producing small quantities of material of high optical purity.
6.4 Kinetic resolution
When a racemic substrate reacts with a homochiral reagent two reactions occur, one with each enantiomer of the substrate. Each process has a transition state which incorporates the same enantiomer of the reagent, but opposite enantiomers of the substrate. The transition states for the two processes have a diastereoisomeric relationship, and the respective activation energies are different. Therefore the reactions proceed at different rates. If the rates of the two reactions are sufficiently different, the reaction can be used for separating the enantiomeric reactants.
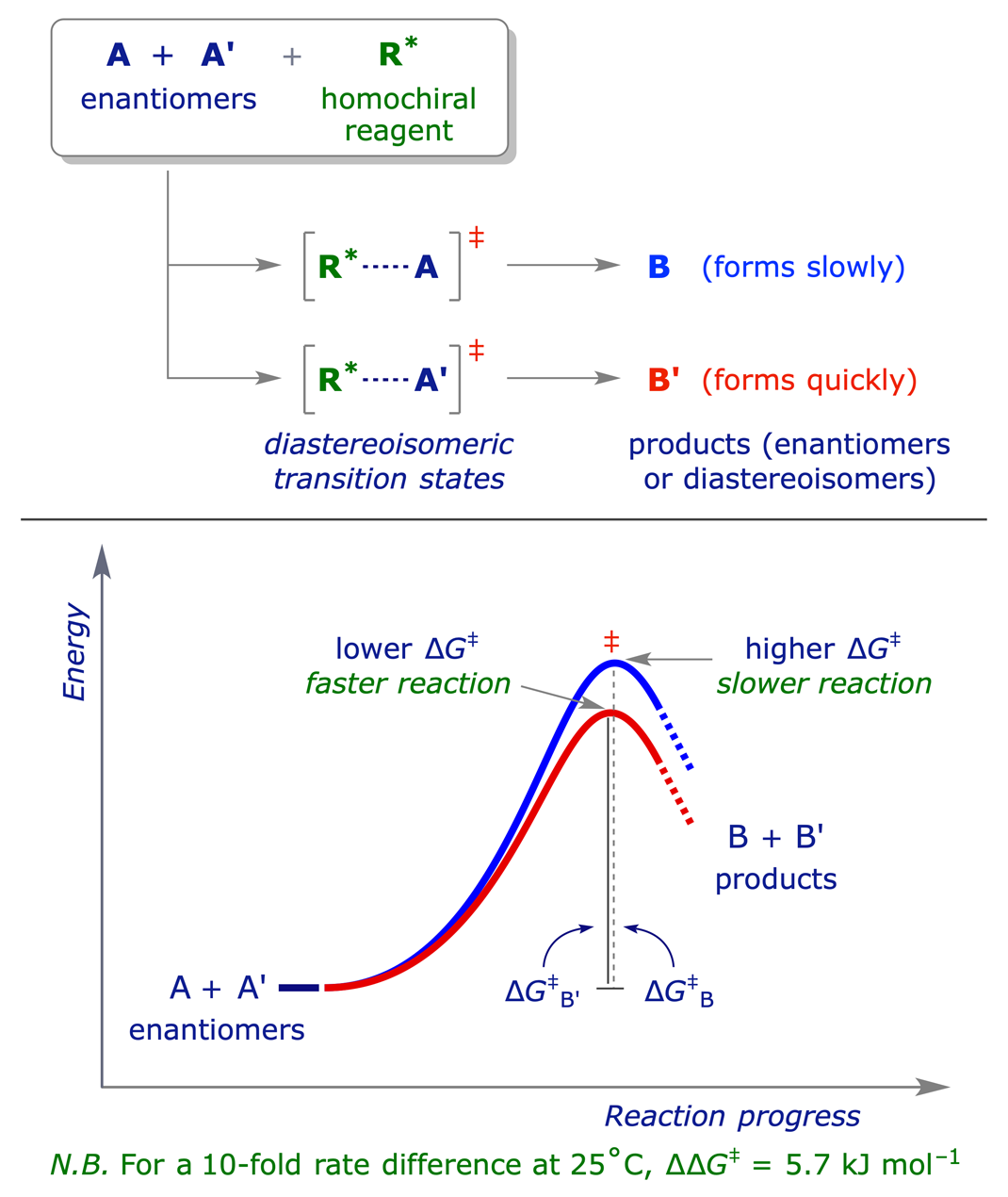
For a kinetic resolution to be 100% efficient, the faster reaction would have to be finished before the slower reaction starts. This is not normally possible but in some cases products of high optical purity can be obtained. There are some chemical processes which provide effective kinetic resolution, but the commonest examples involve the use of enzymes. Being themselves chiral, enzymes can discriminate between enantiomers, and if the diastereoisomeric transition states formed when each enantiomer binds to the enzyme are significantly different in energy, the enantiomers will be 'processed' by the enzyme at different rates.
Ester hydrolysis (or esterification) using an esterase enzyme can be particularly effective, proceeding with high selectivity under mild conditions and at various scales. It is one of the most commonly used kinetic resolution processes.
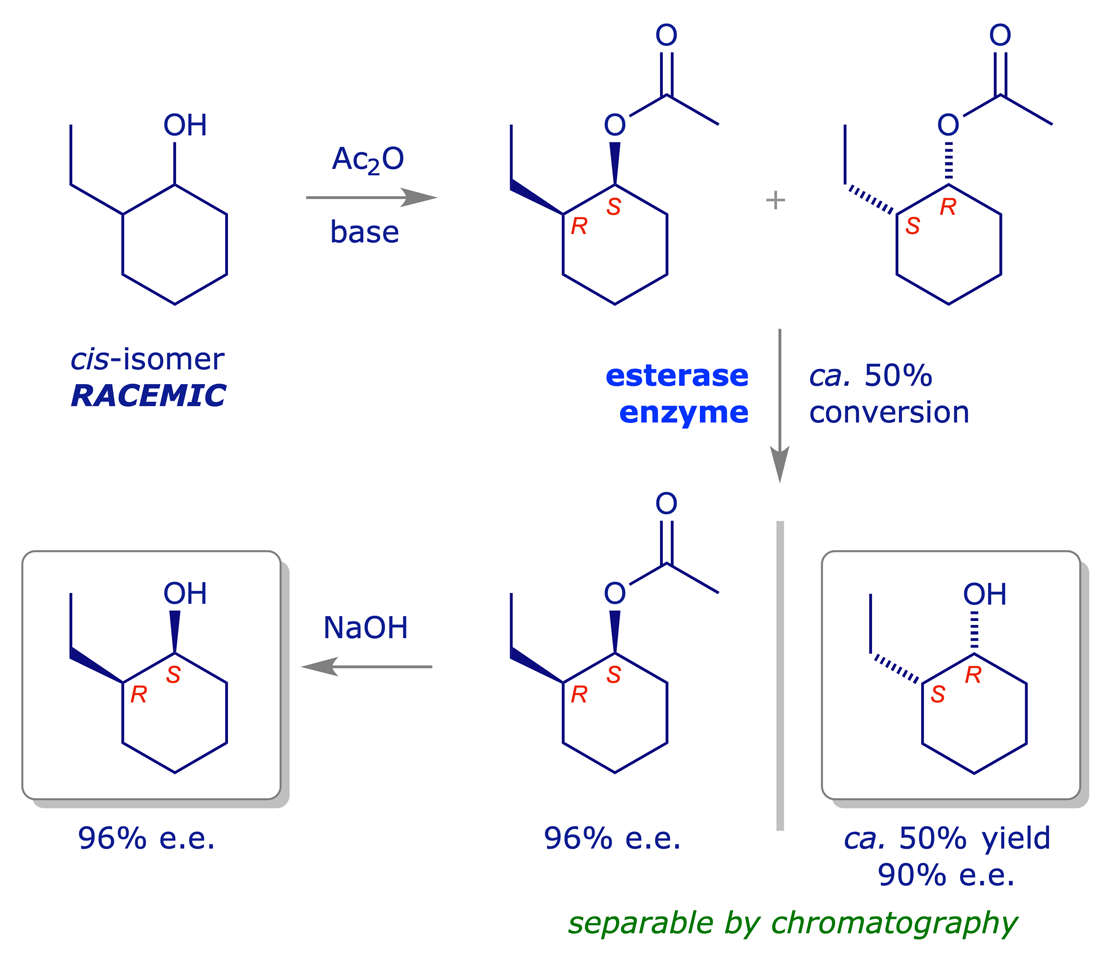
With kinetic resolution processes there is always a compromise between the yield of the required product and its optical purity. For example, if the reaction above is stopped too soon only a small quantity of alcohol will have been formed, but it will be of high optical purity. The remaining material will be unreacted ester of low optical purity. If the reaction is allowed to continue for too long, a large quantity of alcohol will be isolated but its optical purity will be low. However, the unreacted ester will have a high optical purity. It is often worth compromising yield of product for high optical purity.
