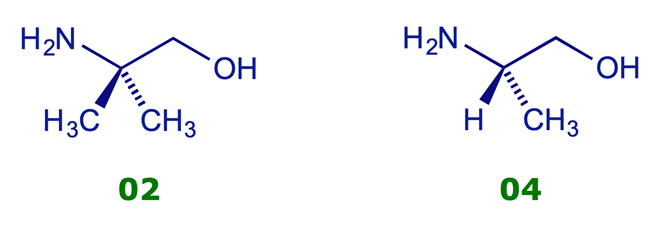5 Diastereoisomers
5.1 Defining features of diastereoisomers
Diastereoisomers are defined as stereoisomers (same constitution, different configuration) which are not enantiomers (i.e. diastereoisomers are neither superimposable nor mirror images of each another). They must have at least two stereogenic centres.
Consider a compound with two stereogenic centres (SCs). If both SCs are inverted, the outcome is the enantiomer of the original compound. But if only one of the SCs is inverted the outcome is a diastereoisomer of the original compound. Notice that if the same SC in each of a pair of enantiomers is inverted, the two products are diastereoisomers of the original pair and enantiomers of each other.
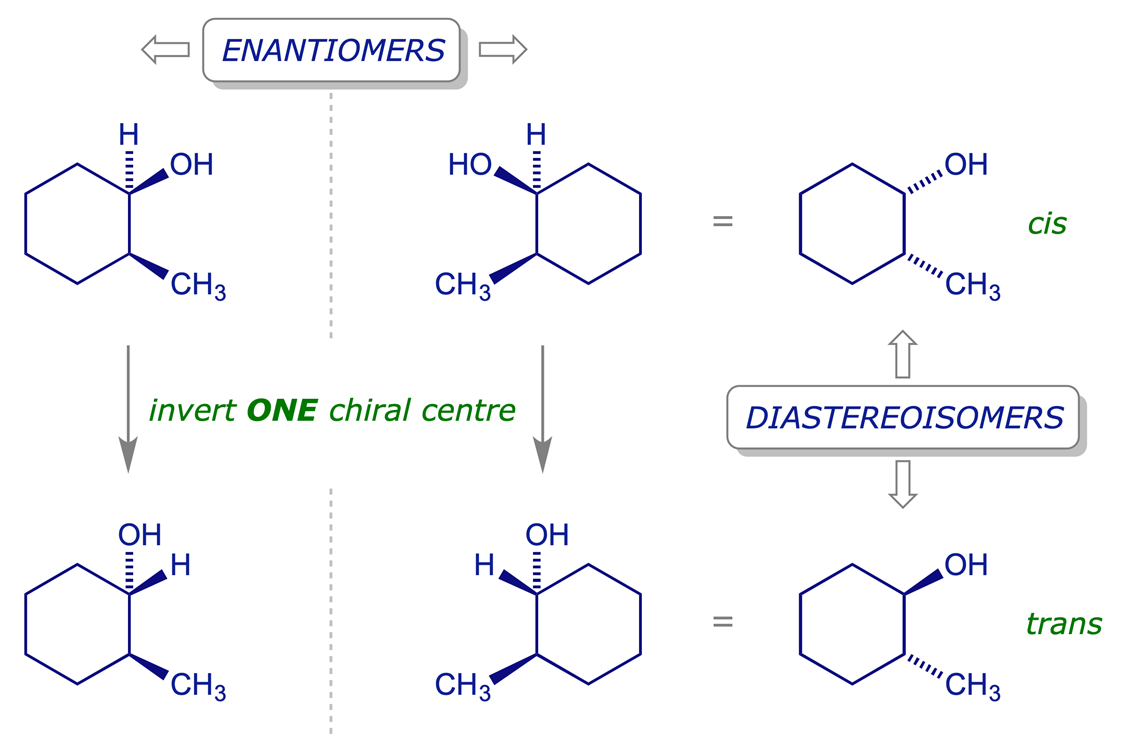
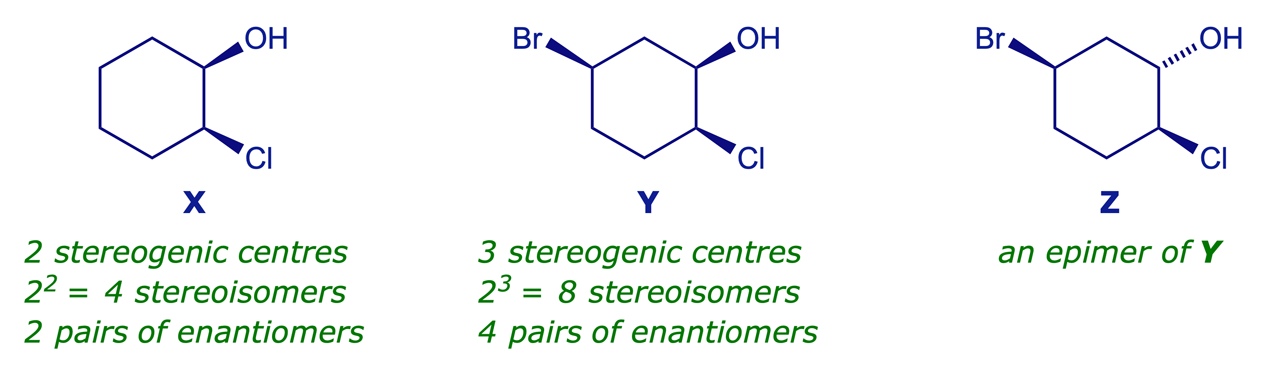
For a structure with n chiral centres there may be up to 2n possible stereoisomers (any meso forms will reduce the possible number of stereoisomers). Each diastereoisomer will have an enantiomeric partner and there are therefore up to 2n−1 pairs of enantiomers. Diastereoisomers that have the different stereochemical arrangement at any one chiral centre are often referred to as epimers.
Diastereoisomers are, essentially, different compounds. They have different physical properties (solubility, m.p., b.p., NMR, IR spectra etc.) and so can be separated by physical methods such as distillation or conventional chromatography (TLC, HPLC, GLC). The relationship between diastereoisomers is fundamentally different to that between enantiomers, which have identical properties (except when interacting with other chiral systems).
5.2 Relationships between atoms (or groups) within a molecule
It is important (and useful) to be able to classify the relationship between different atoms or groups within a molecule. For example, the recognition of chemical and magnetic equivalence of different hydrogen atoms within a molecule is essential when interpreting NMR spectra. For this reason we will focus for a while on relationships between H atoms, but the same principles can be applied to other atoms or groups.
5.2.1 Homotopic hydrogens (or groups)
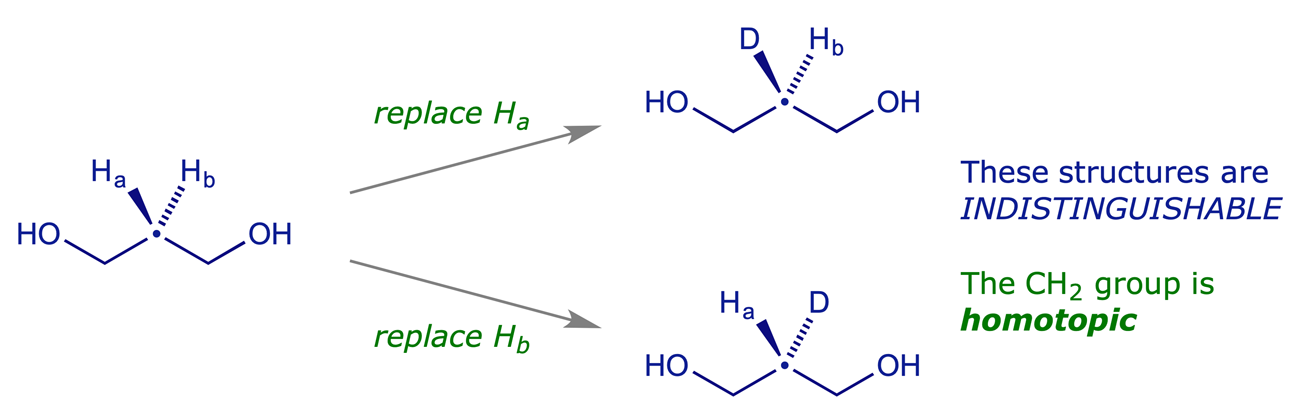
Two hydrogens are homotopic if replacing either one in turn by another group (e.g. D) gives two identical molecules. Homotopic hydrogens are indistinguishable. In the NMR spectrum they are completely equivalent to one another, have the same chemical shift, etc.
5.2.2 Enantiotopic hydrogens (or groups)
Two hydrogens (or other groups) in an achiral or meso compound that are equivalent because of a mirror plane are enantiotopic if replacing one of them with a different group leads to a chiral molecule.
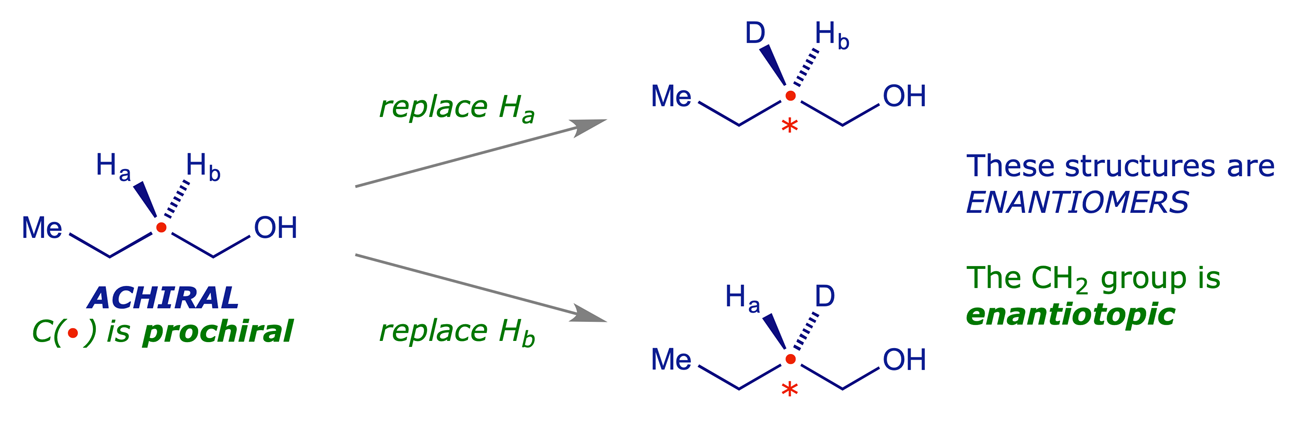
Enantiotopic protons are equivalent in all respects except in a chiral environment. They have the same NMR chemical shifts in achiral media. Chiral reagents, e.g. enzymes, can distinguish between enantiotopic hydrogens (or other enantiotopic groups). For two hydrogens to be enantiotopic they do not have to be attached to the same carbon atom — if making one of the two different from the other removes a plane of symmetry, then they are enantiotopic.
Example:
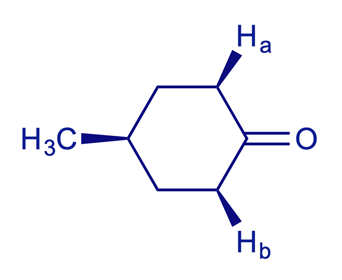
5.2.3 Prochirality
A carbon atom carrying enantiotopic hydrogens is called a prochiral centre: replacing either of the two hydrogens produces a chiral centre. Trigonal (sp2) carbon atoms with three different groups attached are also prochiral — the addition of a new fourth group will generate a chiral centre.
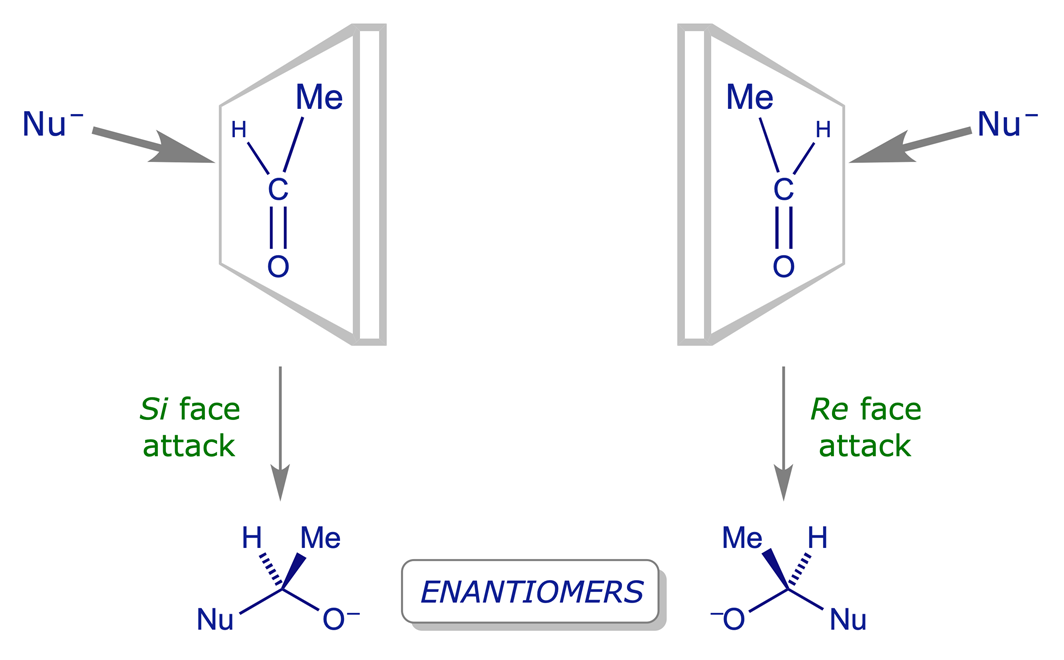
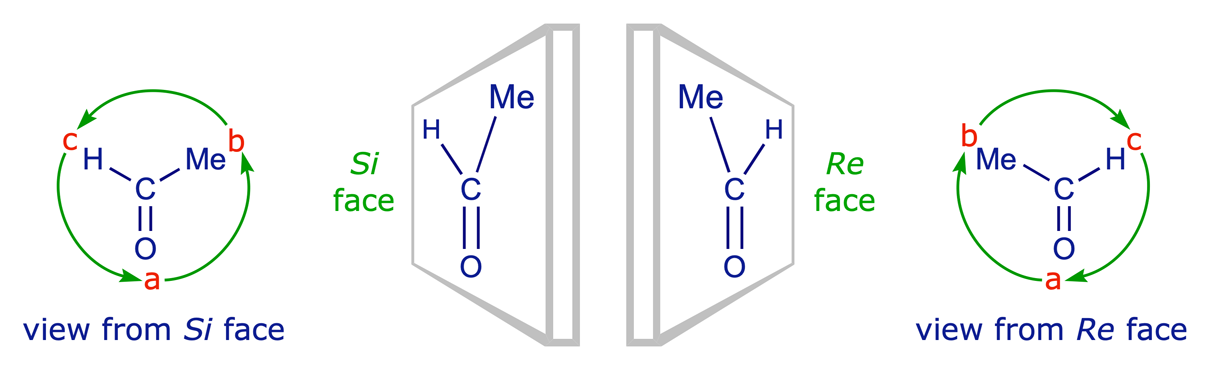
Re and Si nomenclature: This is how the Cahn-Ingold-Prelog system is used to specify the two faces of a prochiral trigonal centre. The priorities a>b>c are determined by the Sequence Rules. The order a to b to c of the ligands is clockwise when viewed from the Re face, anticlockwise when viewed from the Si face.
5.2.4 Diastereotopic hydrogens (or groups)
If the replacement in turn of each of two identical atoms (or groups) attached to a non-stereogenic centre gives different diastereoisomers, the atoms (or groups) are diastereotopic.
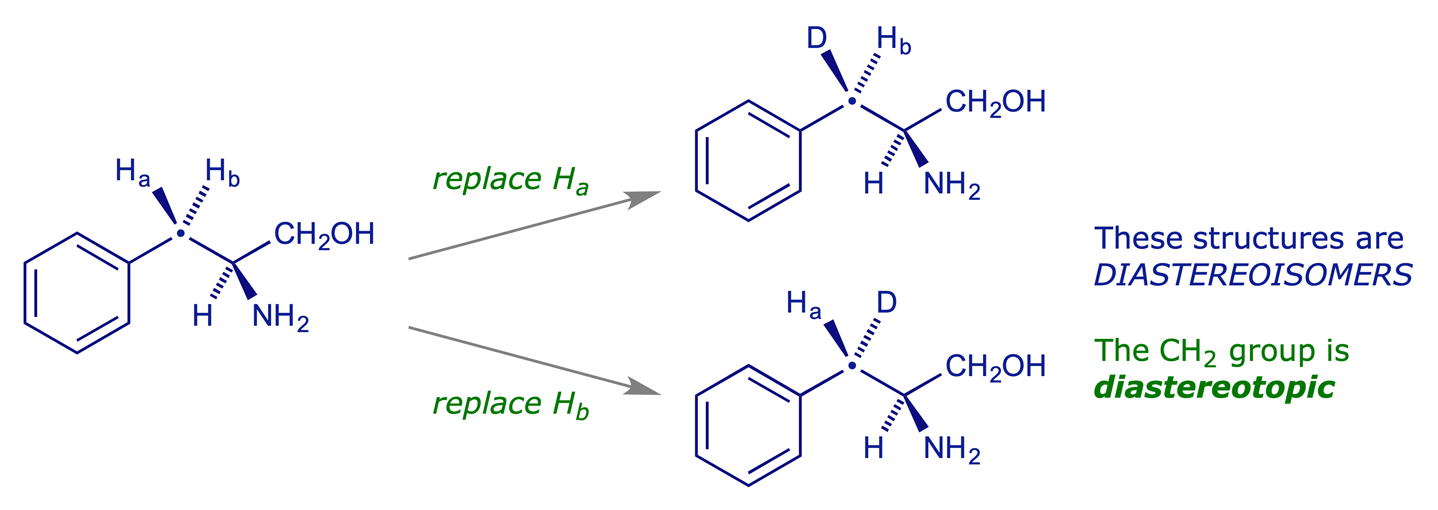
It is important to recognise when a CH2 group is diastereotopic and when it is not. Generally if a molecule does not have a plane of symmetry which bisects the H-C-H angle of a CH2 group, then that CH2 group is diastereotopic. This means that any CH2 unit in a chiral molecule has diastereotopic hydrogens, and the following should be borne in mind.
- Achiral reagents can, in principle, distinguish between diastereotopic hydrogens (e.g. for steric reasons).
- Diastereotopic hydrogens are non-equivalent, so in an NMR spectrum they will appear at different (but possibly similar) chemical shifts. If they are fairly close to a stereogenic centre, their signals may be well separated in the spectrum. The difference in chemical shift usually diminishes with distance from a chiral centre, and might even become zero, but diastereotopic hydrogens are never equivalent.
- Because diastereotopic hydrogens have different chemical shifts, they can undergo spin-spin coupling to each other.
Compare the different CH2 signals in the following NMR spectra, shown in Appendix A (PDF, opens in new window):
02 2-Amino-2-methyl-1-propanol
04 (S)-2-Amino-1-propanol