Methylcyclohexane
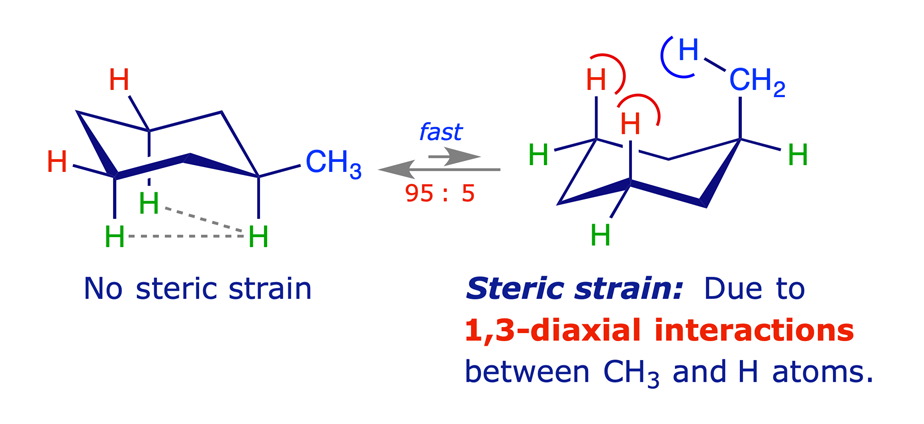
The most stable conformation of methylcyclohexane is the chair conformation in which the methyl group is equatorial. The alternative chair conformation, in which the methyl group is axial, is 7.3 kJ/mol higher in energy. This difference corresponds to a equatorial:axial conformer ratio of 19:1 at 25 °C.
×
![]()
Use the interactive model below to explore the conformations and ring flipping of methylcyclohexane. In particular, witness for yourself the destabilising 1,3-diaxial steric interactions using the following combination of settings:
reset → Atoms 100% → rotate 120° → colour atoms → ring flip continuous
Watch what happens to the methyl group and the two hydrogens as the ring flips.
Go to cyclohexane
Go to disubstituted cyclohexanes
