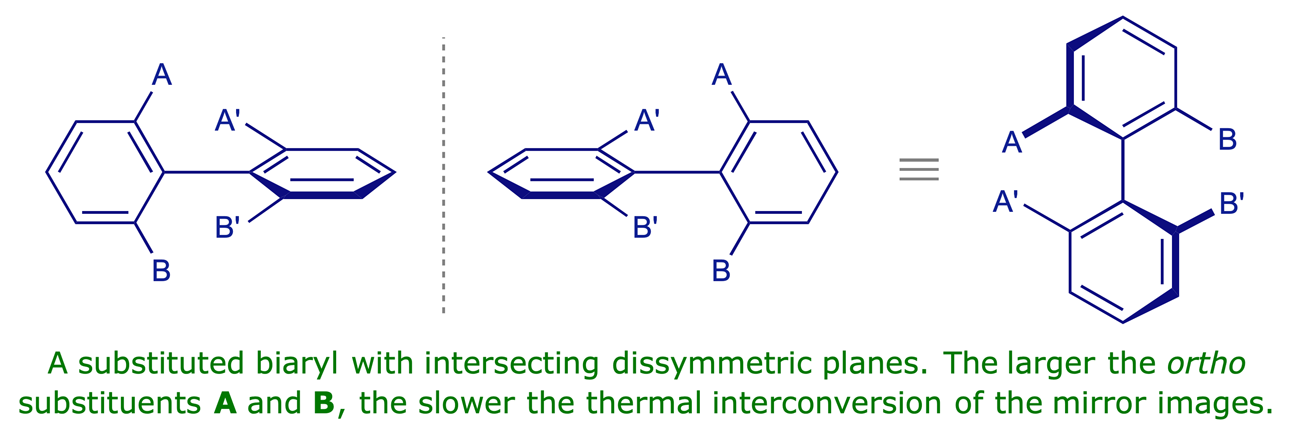
Atropisomerism in biaryls
Atropisomers ('non-turning isomers') are enantiomeric conformations that are separable because rotation around a single bond is sterically hindered. This phenomenon is encountered in substituted biaryls. As in the case of allenes, the crucial geometric feature in such compounds is dissymmetric perpendicular planes, neither of which is a plane of symmetry.
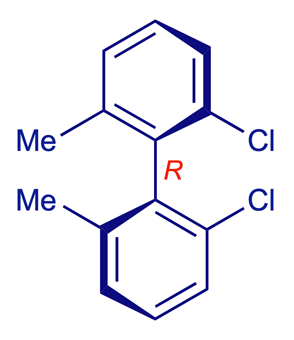
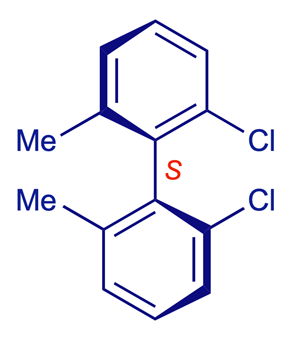
2,2'-Dichloro-6,6'-dimethylbiphenyl
A simple pair of stable atropisomers is shown below. Although the two aromatic (aryl) groups are connected by a single C–C bond, rotation about this bond is prevented by the sheer size of the methyl and chloro substituents in the positions next to the inter-aryl bond (select Atoms 100% for a visual impression of the steric effects involved). Consequently the two conformations shown do not interconvert at room temperature and can be isolated separately. They are enantiomers, i.e. non-superimposable mirror images.
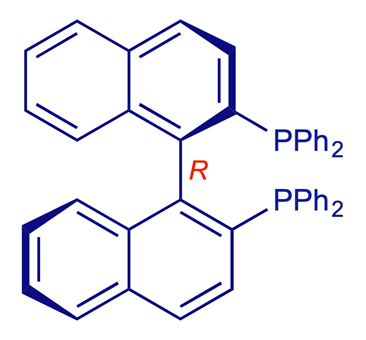
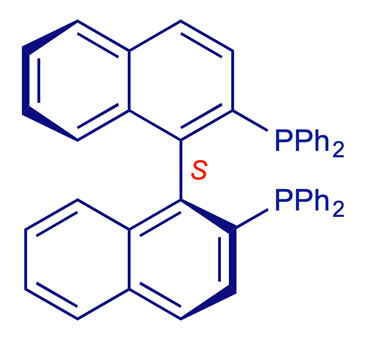
BINAP [2,2'-bis(diphenylphosphino)-1,1'-binaphthalene]
The 1,1'-binaphthalene compound BINAP is a commercially important biaryl. BINAP is a rigid, chiral bidentate phosphine ligand, available in both enantiomeric forms and widely used in laboratory and plant (industrial) processes, e.g. the transition metal-catalysed enantioselective hydrogenation of C=C and C=O double bonds. The 2001 Nobel prizewinner Ryoji Noyori pioneered the use of biaryls such as BINAP in catalytic enantioselective synthesis. To simplify the models below, the large PPh2 groups are represented as single orange-coloured atoms.