3 Stereogenic axes
3.1 Achiral molecules with chiral centers?
3.1.1 Are you analysing it CAREFULLY?
Some molecules may at first appear to have a stereogenic centre, but on close inspection, two of the groups attached to it are found to be identical.

3.1.2 Meso compounds
In the previous section we saw that some molecules incorporate stereogenic centres but also have a plane or centre of symmetry which causes the molecule as a whole to be achiral. One stereoisomer of 2,3-dichlorobutane is a meso compound (it has stereogenic centres and a plane of symmetry).
In section 2.1.4 we saw that some molecules have stereogenic centres but also have a plane or centre of symmetry which causes the molecule as a whole to be achiral. One stereoisomer of 2,3-dichlorobutane is a meso compound (it has stereogenic centres and a plane of symmetry).
3.1.3 Disubstituted cyclohexanes
Some are chiral, others are not!

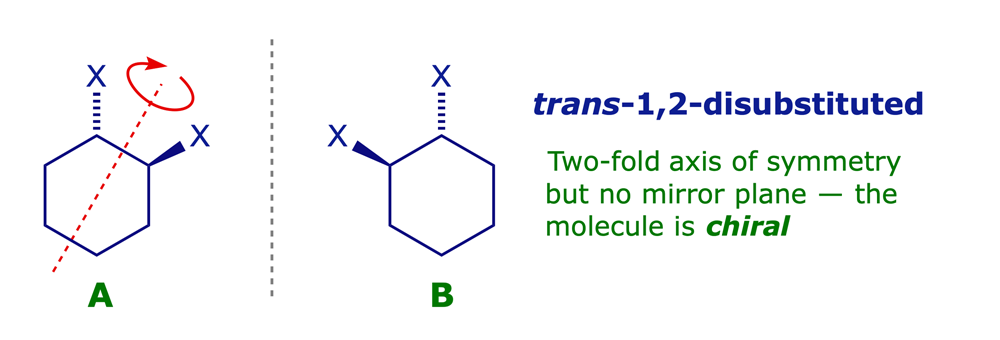
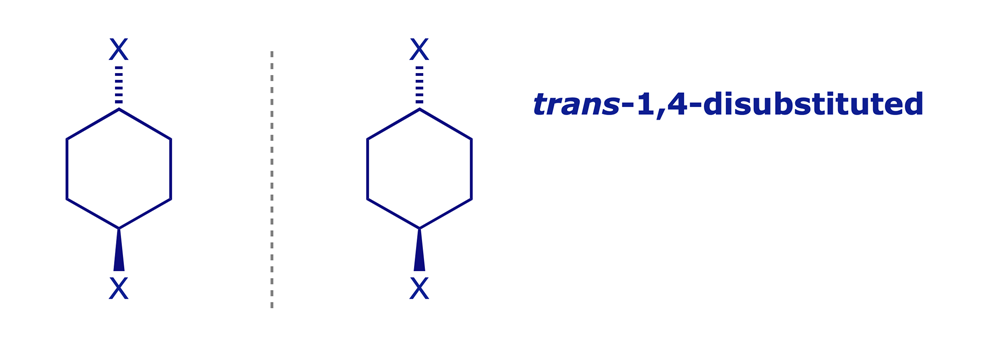
3.1.4 Acyclic systems
Interactive models
For acyclic systems all possible conformations need to be considered and compared with their mirror images.
![The structures of (<em>R</em>,<em>S</em>)- [<em>meso</em>], (<em>S</em>,<em>S</em>)- and (<em>R</em>,<em>R</em>)-bis(1-phenylethyl)amine](images_SC/3005.png)
3.1.5 'Chiral' centres at a 'pseudo' C2 axis of symmetry
Interactive models
A trisubstituted tetrahedral atom at a position which, without its third substituent, would be on a C2 axis of symmetry, is not stereogenic.

In the above molecule, it does not make any difference whether you draw the X group as receding or emerging — the two alternatives are identical.
3.1.6 Compounds with a centre of symmetry
Compounds with a centre of symmetry are quite rare. They do not have a mirror plane, but are identical with their mirror images and are therefore achiral. Thus A and B are exactly the same structure.

3.2 Chirality without chiral centres
There are several classes of chiral compound which do not possess stereogenic atoms. The most important are those with intersecting dissymmetric planes. The structure of the important antibiotic vancomycin contains multiple examples of three different types of chirality (central, axial, planar).
3.2.1 Substituted allenes
An allene whose terminal carbons each bear two different substituents (A and B in the example shown) is a structure with fixed intersecting dissymmetric planes and is therefore chiral (the mirror images are non-superimposable). The simplest example is 2,3-pentadiene (A = H, B = CH3).
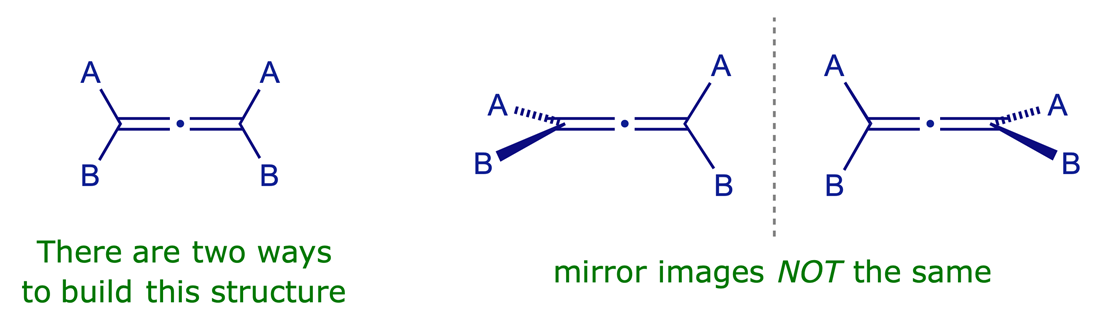
3.2.2 Atropisomers
Interactive models
Atropisomers are enantiomeric 'conformations' that are separable because of sterically hindered rotation around a single bond. This situation is prevalent in substituted biaryls. Again, the crucial structural feature is intersecting planes, neither of which is a plane of symmetry.
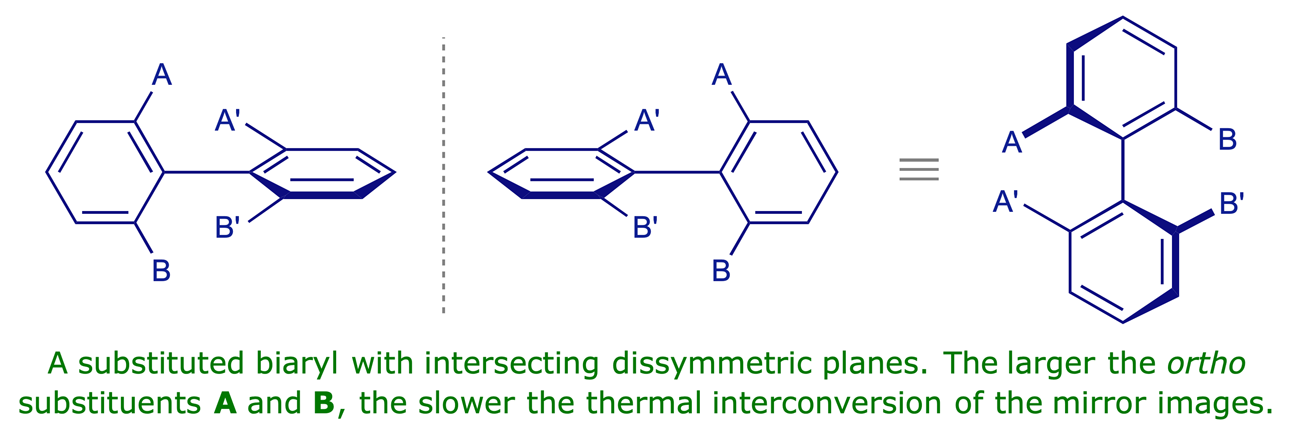

You can also see an animation which illustrates the conformational restrictions in a substituted biaryl.
3.2.3 Helicenes
Interactive models
Some molecules have a molecular skeleton which is inherently chiral (helical), an example being hexahelicene. One side of this molecule has to lie above the other due to steric crowding — if hexahelicene were planar the red-dotted carbon atoms would be in the same place.
![Alternative representations of [6]helicene (hexahelicene)](images_SC/3011.png)
3.2.4 Applying (R,S) stereochemical descriptors to biaryls
Interactive models
The Cahn-Ingold-Prelog system includes procedures for assigning stereochemical descriptors to molecules with axial chirality. The example below is the chiral 'biphenic' acid derivative, 6,6'-dinitrobiphenyl 2,2'-dicarboxylic acid.

- The molecule is viewed in the conformation with the two aryl rings perpendicular, along the axis of the Ar–Ar bond.
- The four relevant substituents are projected onto a plane at right angles to the axis and the groups are assigned priorities as though the projection represents a stereogenic atom. It is immaterial whether the nearest ring is placed horizontally or vertically.
- The projected atoms or groups may not all be different so the sequence rule is augmented with a proximity rule, which specifies that near groups precede far groups.
For example, in the enantiomer of 6,6'-dinitrobiphenyl 2,2'-dicarboxylic acid shown above, the vertical (near) groups take precedence over the horizontal (far) groups. Since NO2 precedes CO2H (sequence rule), the rear CO2H has the lowest priority d and the remaining groups are prioritised a, b and c as shown, using the Cahn-Ingold-Prelog system.
- The configuration is (S) because the sequence a to b to c describes an anticlockwise turn.
In general for assigning the configuration of a biaryl axis, two pairs of substituents (one pair on each ring, as close together as possible are chosen, such that the members of each pair can be distinguished by the sequence rule. For example, compound X is (R.

3.2.5 Applying (R,S) stereochemical descriptors to allenes
Interactive models
The procedure for assigning (R,S) stereochemical descriptors to allenes is similar to that for biaryls. The molecule is viewed along its axis and the relevant substituents are projected on to a plane at right angles to the axis. The groups are prioritised using the sequence rules augmented by the proximity rule, which specifies that near groups precede far groups.

The configuration of (+)-2,3-pentadiene is (S) because the sequence a to b to c describes an anticlockwise turn.
