4 Enantiomers
4.1 Central chirality at atoms other than carbon
Several elements other than carbon can exist in the form of a tetrahedral shape, and are therefore equally capable of chirality in appropriate circumstances.
4.1.1 Other group IV elements
Other elements in group IV, for example silicon (Si) and tin (Sn), form tetrahedral structures in the same way as carbon and they can exhibit chirality if four different groups are attached.
4.1.2 Group V elements
Amines: Nitrogen normally has a valency of three and is pyramidal, but it can be considered as being tetrahedral if we include the lone pair of electrons. If three different groups are attached the nitrogen is, in principle, a stereogenic centre. In practice, for 'normal' amines at room temperature the two arrangements are in equilibrium and the stereochemistry rapidly inverts via a planar sp2 nitrogen atom.
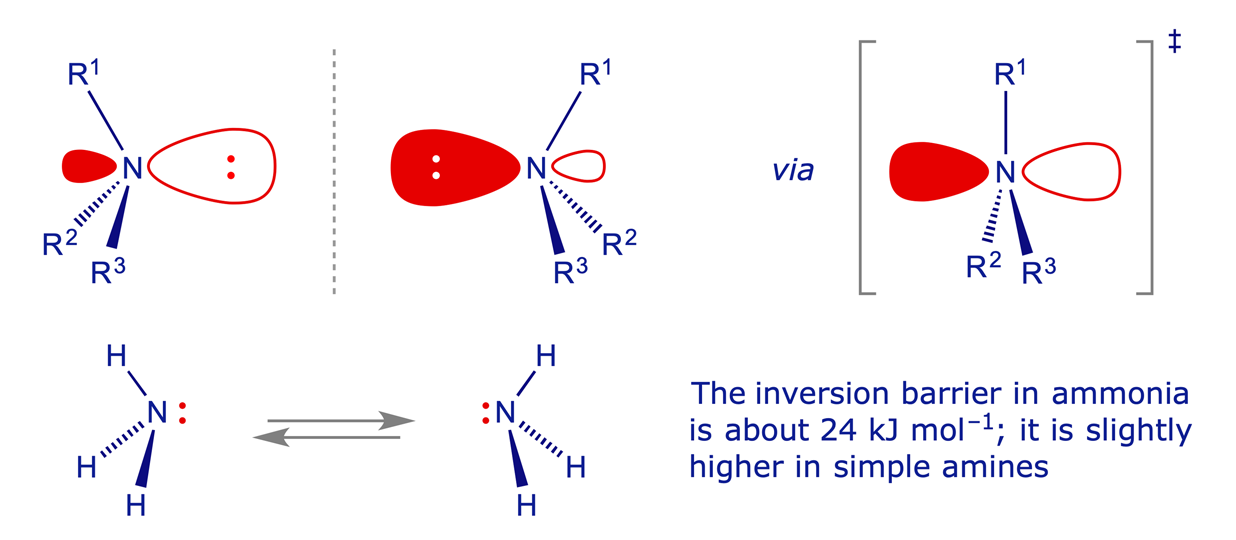
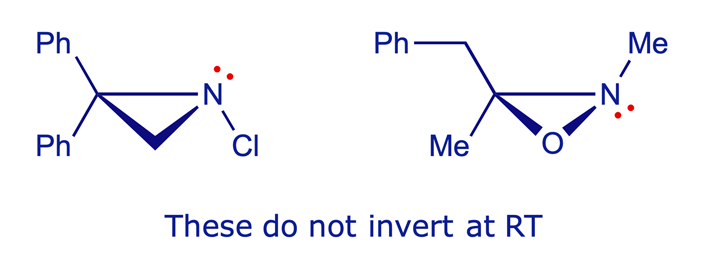
The N inversion barrier is lowered by conjugation with pi-electron withdrawing groups, which stabilise the transition state (in which the N lone-pair is in a pure 2p orbital) by delocalisation. The N inversion barrier is raised when the N is incorporated in a three-membered ring, due to high strain in the transition state, where the N is sp2 but the bond angles are necessarily constrained to 60°. The inversion barrier is also raised by electronegative substituents such as O or Cl, because they reduce the energy of the lone-pair orbital. The inductive withdrawal of electron density from N through the N–O or N–Cl sigma bond has a knock-on effect, in that the N lone-pair electrons in their sp3 orbital become more tightly bound to the nucleus. This widens the energy gap from the sp3 hybrid orbital to the pure 2p orbital of the transition state structure. The molecules shown below illustrate these principles.
Kinetics equations and calculator
Inversion kinetics
To put things into perspective....
If you assume that ∆S is zero, you get these half-lives for inversion at 25 ˚C:
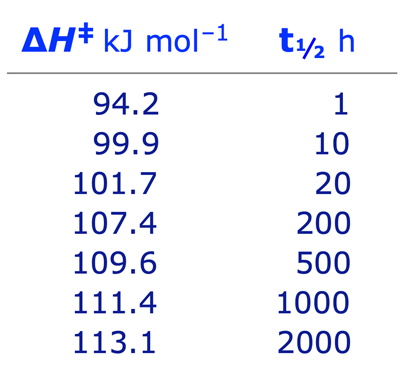
For other temperatures, half-lives will halve (approx.) for every 5K added.
Data and models
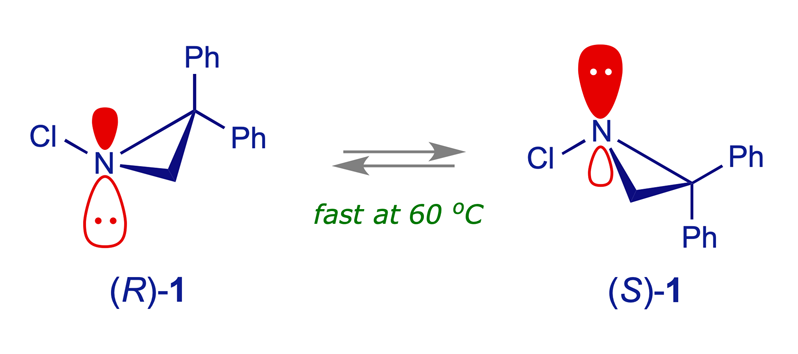
The kinetics of nitrogen inversion for the aziridine 1, shown in the table below, were determined in the 1980s by an Italian group, who also published the compound's crystal structure. They observed the expected first-order kinetics for racemisation of (R)-1, and that the height of the activation barrier is relatively insensitive to the solvent.
Data and models
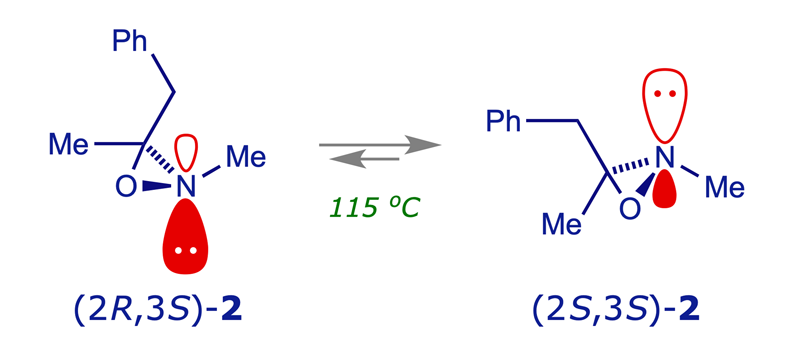
Nitrogen inversion in the oxaziridine 2 was studied by a German group. The nitrogen was configurationally stable at normal temperatures but inversion became prominent on heating above 100 °C. Note that the two 'invertomers' 2a and 2b are not identical and that the equilibrium favours 2b (why?).
N-Oxides and ammonium salts: In N-oxides and ammonium salts the tetrahedral arrangement at nitrogen is effectively locked (the inversion barrier is very high) and if four different groups are attached it is a chiral centre.

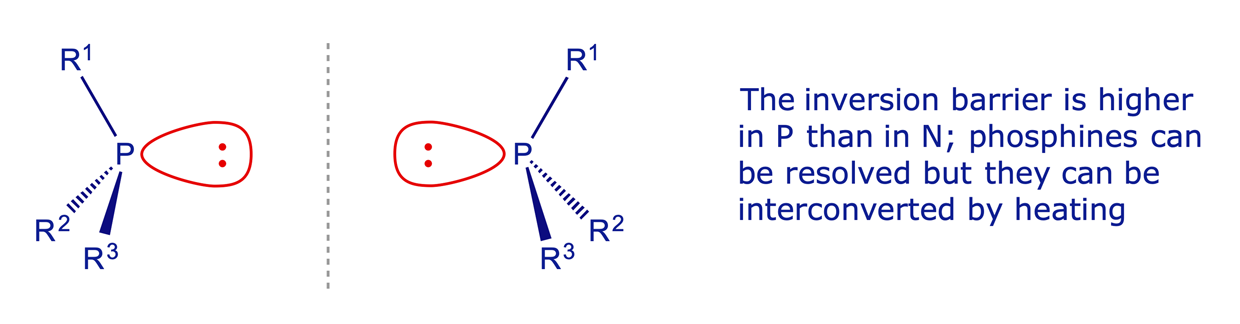
Phosphines: In common with the nitrogen atom in amines, phosphines have a tetrahedral P atom and are chiral if three different groups are attached to it. The P atom can invert by the same mechanism as amines but the activation barrier is higher and the enantiomers can sometimes be resolved. However, they are readily interconverted by heating.
Data and models
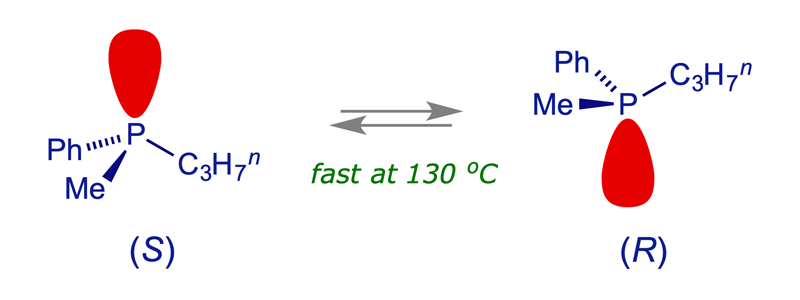
The thermal racemisation of optically active methylpropylphenylphosphine was studied in the 1960s. The half-life, which varied slightly with solvent, was a few hours at 130 °C.
4.1.3 Group VI elements
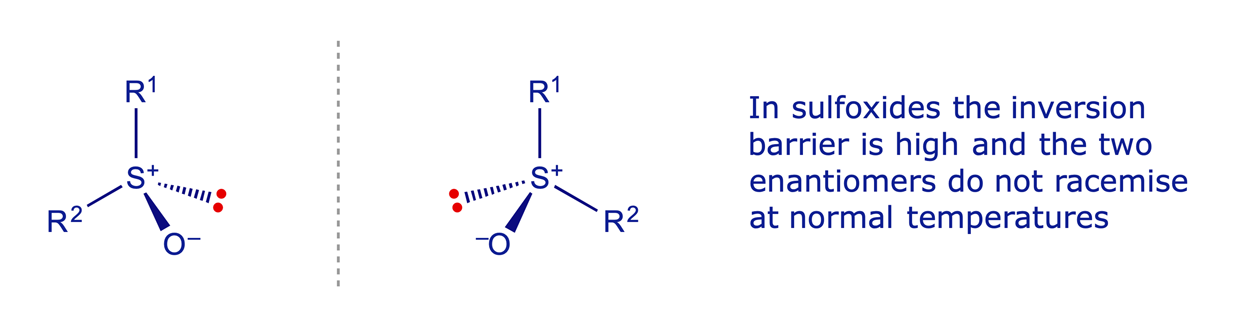
Sulfides (and ethers) are planar, but the sulfur atom in sulfoxides, sulfones and sulfates is tetrahedral and configurationally fairly stable. A sulfoxide with two different groups attached therefore has a stereogenic sulfur atom.
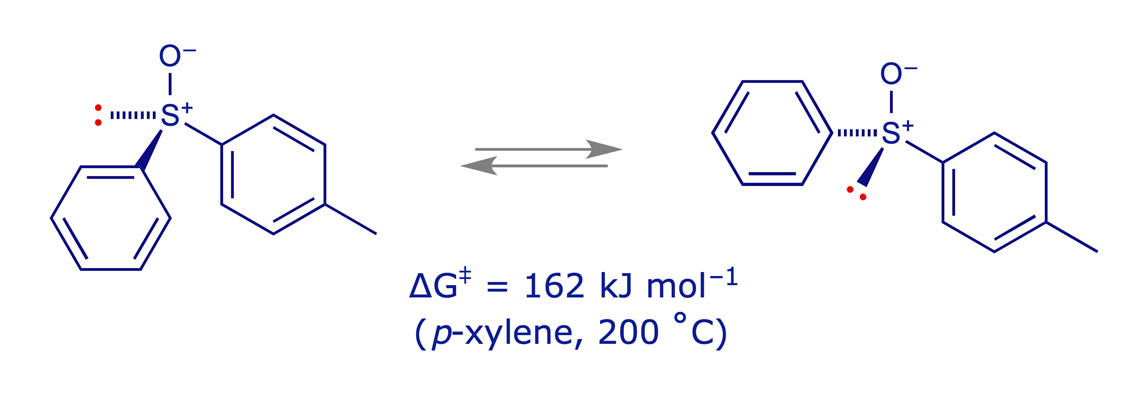
There are some very effective chiral reagents based on sulfoxides. Their main drawback is the difficulty of producing them in enantiomerically pure form.
4.2 Properties of enantiomers
All electronic, steric and other geometric interactions within one enantiomer are identical to those in its mirror image — therefore the general physical properties of two enantiomers are identical. These include m.p., b.p., all spectra (NMR, IR etc.), TLC, Rf values etc. They also have the same general chemical properties (pKa, reaction rates etc.).
Exception: Enantiomers can show different properties in a chiral environment. A chiral environment might be provided by interaction with other chiral molecules or biomolecules (see later for more detail).
4.2.1 Optical activity
Enantiomers rotate the plane of polarisation of plane-polarised light to an equal extent, but in opposite directions. This phenomenon is measured using a polarimeter.
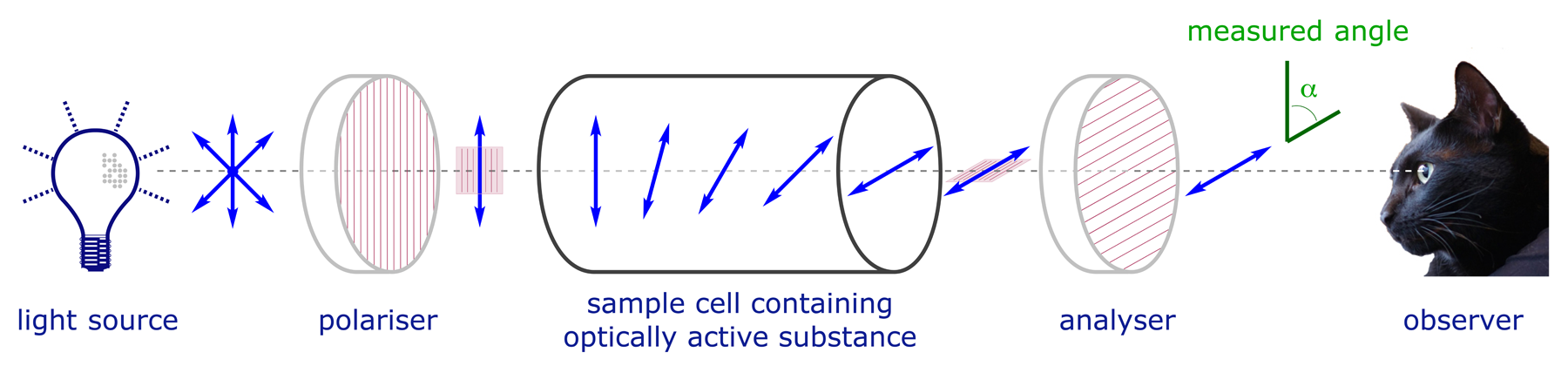
To define a standard optical rotation measurement (the specific rotation) for a particular compound we standardise for unit concentration (grams per c.c.) and unit path length (one decimeter) using the following equation:

where
- [α]D is called the specific rotation of the compound
- The units of [α]D are 10−1 deg cm2 g−1 (usually omitted; often given wrongly as 'degrees')
- The letter 'D' refers to the sodium D line (wavelength 589 nm)
- The superscript T is the temperature in °C of the measurement (usually 20)
- For a neat liquid, c (g cm–3) is the same as the density
- Compounds with clockwise (+) rotations are dextrorotatory
- Compounds with anticlockwise (−) rotations are laevorotatory
4.2.2 Biological activity
There are many types of small molecules, for example hormones (chemical messengers), drugs, poisons, etc., which have powerful biological activity resulting from their interactions with larger 'receptor' molecules. Most receptor sites in mammals are proteins, and since these are built from chiral amino acids, proteins are also chiral. The interaction between two different enantiomers and the same receptor is therefore diastereisomeric, and the two alternative enantiomer–receptor combinations have different binding energies. For example, the antihistamine dexchlorpheniramine is highly stereoselective, the (S)-enantiomer being about 200 times more potent that the (R)-form. The higher potency suggests more complementarity ('a better fit') with the target receptor. Interestingly, the natural substrate for this receptor, i.e. histamine, is itself is achiral.
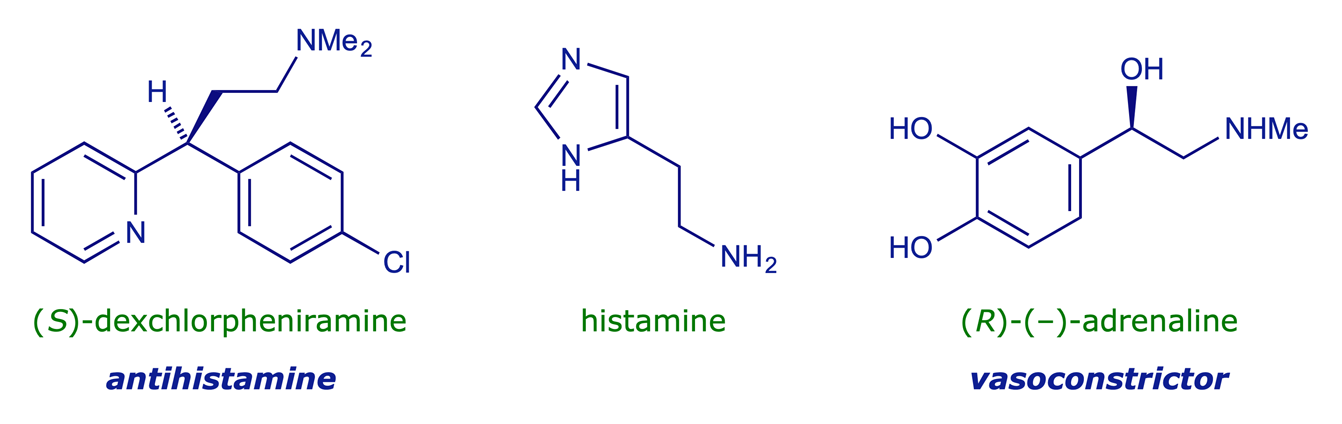
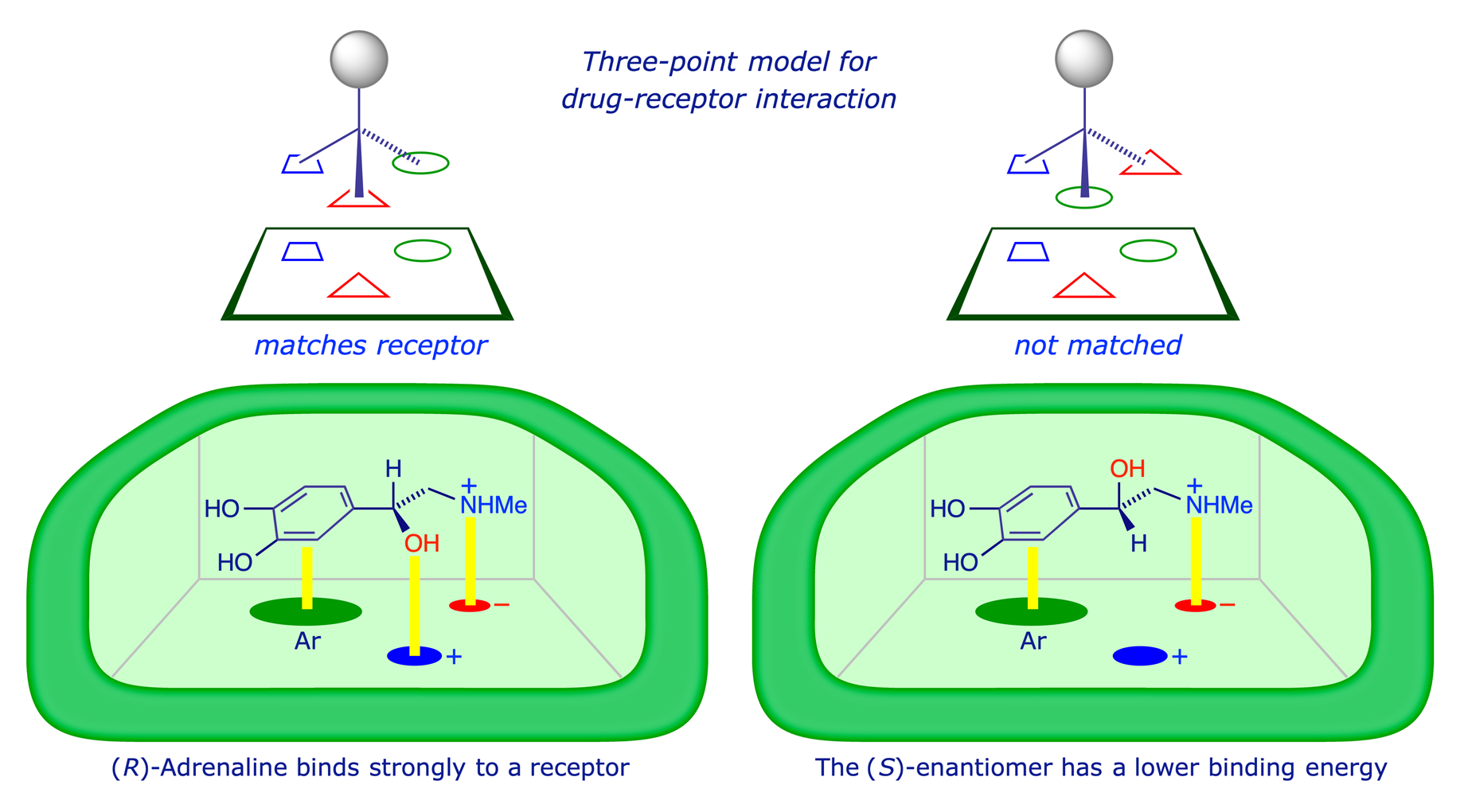
It was recognised some 70 years ago that only three binding sites are required for a receptor to be able to differentiate between two enantiomers. This 'three-point attachment' concept is shown schematically below. The hormone adrenaline mediates vasoconstriction (inducing increased blood pressure and heart rate), but the (R)-enantiomer is more active than the (S)-enantiomer.
Enantiomers can have biological activities which are altogether different. One enantiomer of a biologically active molecule may cause a particular biological response, whereas the other enantiomer is usually completely inactive. It is therefore wasteful, at best, to synthesise and use a racemic mixture of stereoisomers as a drug. However, the 'wrong' enantiomer of a drug may be biologically active in a completely unwanted way, which can be very dangerous. The most infamous example of this is the sedative thalidomide, the (S)-enantiomer of which is teratogenic.
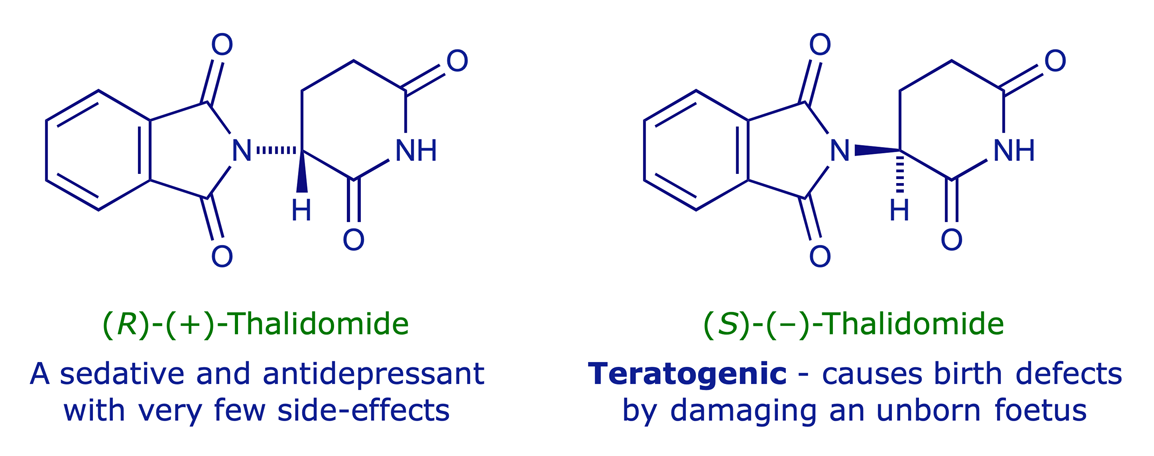
This provides the clearest indication that, if we are to make use of the biological properties of chiral molecules, we need to be able to prepare them as single enantiomers — which is not always easy.
