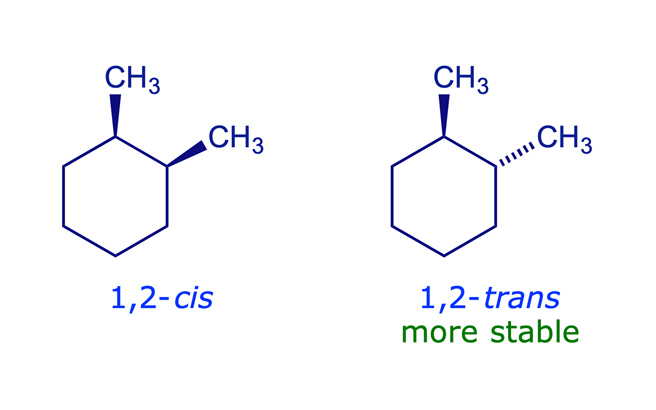
1,2-Dimethylcyclohexane
The preferred chair has both methyls equatorial, which minimises 1,3-diaxial repulsions. In the 1,2-disubstituted case this is possible only for the trans-isomer, which is 6 kJ/mol more stable than the cis-isomer (in the 1,2-cis isomer one of the methyl groups must be axial).
×
![]()
Go to methylcyclohexane
Go to dimethylcyclohexanes
Go to 1,3-dimethylcyclohexane
Go to 1,4-dimethylcyclohexane
