Dr Tim Wallace

Honorary Senior Lecturer
Location: G.025 Dover Street Building
Email: tim.wallace@manchester.ac.uk
Research Themes: Synthesis, Life and Health
Biography
BSc (1971), PhD (1974), Imperial College, London
Postdoctoral research: Geneva (1974–1976); Sussex (1976–1980)
Lecturer/Senior Lecturer, Salford (1980–2001)
Senior Lecturer, UMIST and Manchester (2001–2015)
Research Fellow in eLearning, Manchester (2015–2017)
Honorary Senior Lecturer, Manchester (since 2017)
Online Teaching Modules
Research Interests
The group's focus has been the development and application of organic synthetic methods, including the metal-free radical cyclisation of unsaturated ketoesters, stereoselective electrocyclic and cycloaddition reactions, and asymmetric induction using chiral auxiliaries. Targets of interest include anticancer and antifungal agents, antimalarials, naturally occurring polyenes and various terpenoids. In each area the aim has been effective synthetic processes and the provision of biologically active materials for testing.
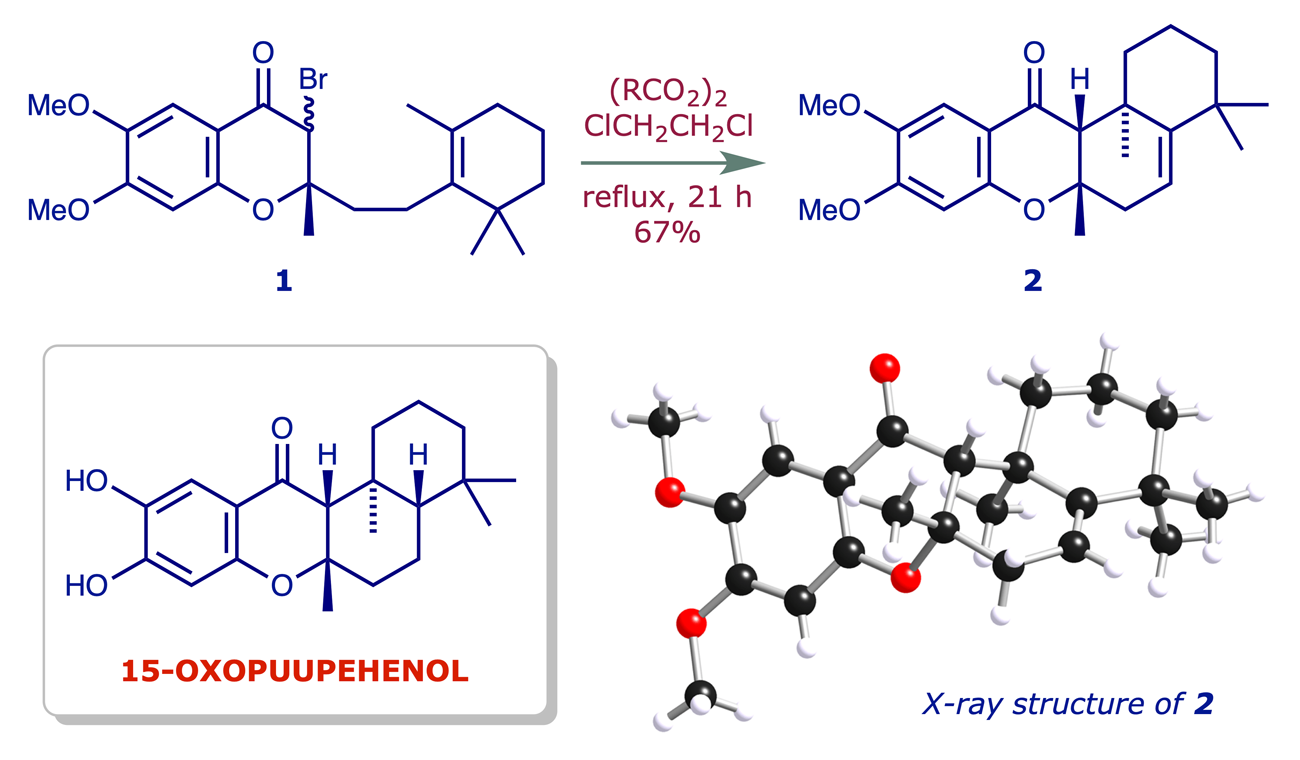
Marine natural products: In the course of studies on radical cyclisation reactions we showed that the bromochromanone 1 can be efficiently cyclised to the tetracyclic structure 2. This reaction is seen as a potential source of analogues of the sponge metabolite 15-oxopuupehenol, which has antimalarial properties (ref. 1).
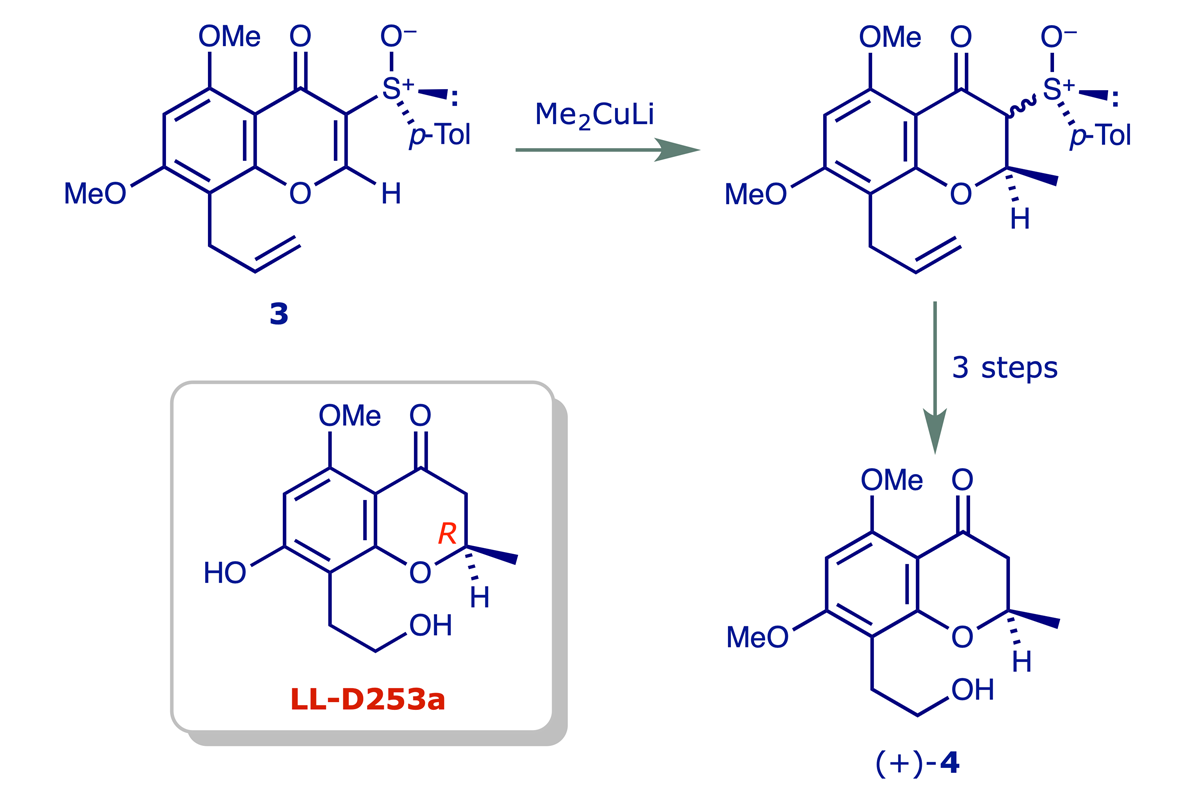
For the synthesis of homochiral 2-substituted chromanones we developed a conjugate addition strategy in which a sulfoxide group serves as a chiral auxiliary. Thus the derivative 4 of the chromanone antibiotic LL-D253a was prepared in high optical purity from 3 as shown below, and the assignment of a 2R configuration for the natural product was thereby confirmed (ref. 2).
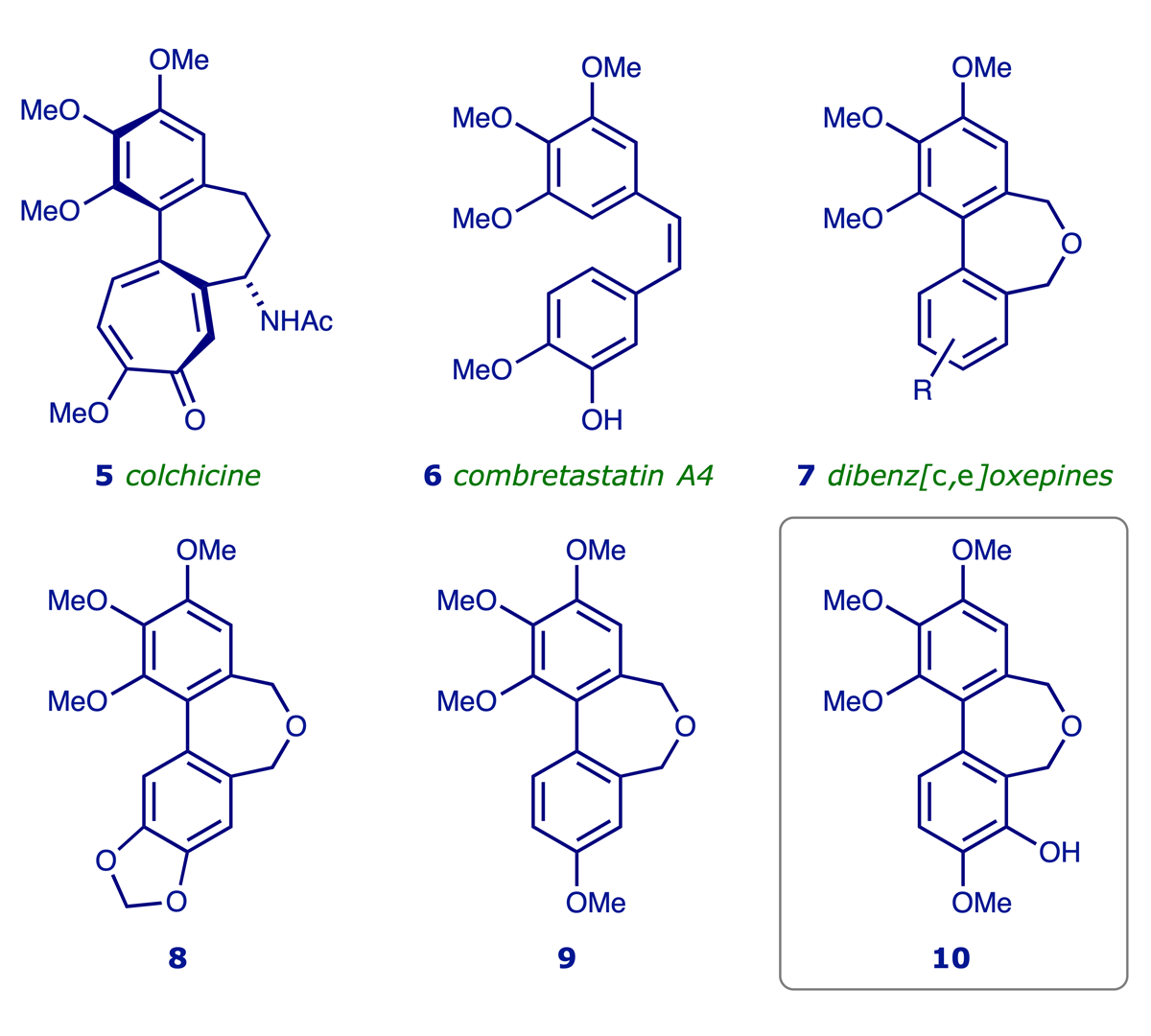
Tubulin-Inhibiting Novel Heterocycles for Anticancer Therapy (the TINHAT project): In a project directed towards the development of new anticancer agents, we synthesised a series of heterocycles related to the alkaloid colchicine 5. Although colchicine is well known for its ability to bind to the protein tubulin and thereby disrupt cell division, it is too toxic for clinical use. However, it has been recognised that less toxic small molecules with tubulin-binding ability may be useful as vascular disrupting agents (VDAs). One of the pioneering VDAs is combretastatin A4 6, which was isolated from the African bush willow, Combretum caffrum, and subjected to various clinical trials. This simple compound selectively targets the microtubular structures in newly-formed tumour-associated blood vessels, with the effect of blocking the flow of blood to the tumour, thereby depriving it of oxygen and nutrients. Our own work in this area led to the identification of heterocycles of the form 7 as a source of new tubulin binding agents, and the compounds 8–10 were found to possess significant tubulin binding ability and in vitro cytotoxicity. We have demonstrated that 10, administered as the corresponding water-soluble phosphate derivative, induces vascular shutdown, necrosis and growth delay in tumour xenografts in mice at sub-toxic doses (ref. 3). The TINHAT project, originally supported by the Association for International Cancer Research and The University of Manchester's commercial arm UMIP (now Innovation Factory), is the subject of an international patent (ref. 4).
Cyclobutene chemistry: The selection rules which underpin electrocyclic reactions make them a valuable source of stereoselectivity, and our studies with cyclobutenes revealed some new aspects of the chemistry of these compounds. Aldehydes such as 11 undergo ring opening not only at low temperature but also with complete stereoselectivity, giving only 2Z,4E-dienals 12. We developed a new multicomponent reaction that provides cyclobutanes 13 from three starting materials in a single step, making substituted cyclobutenes of the form 14 readily accessible in a mild, flexible and stereoselective manner (ref. 5).

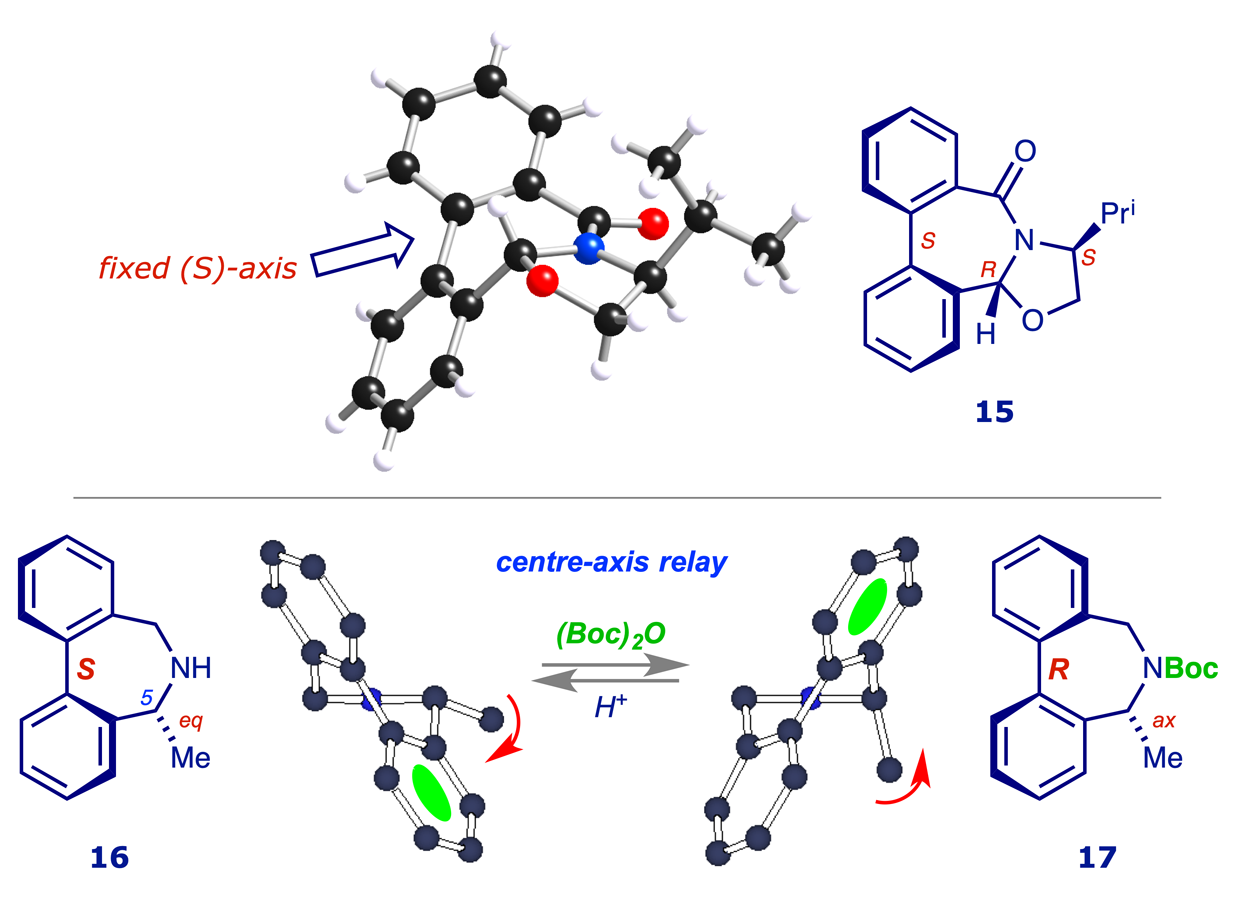
The control of axial chirality: There is much interest in synthetic routes to molecules possessing axial chirality (ref. 6), and we prepared the first biaryl-fused oxazolidine lactam 15 in which the axis is conformationally locked (ref. 7). We also converted a related lactam into the previously unknown secondary amine 16, in which the stereogenic axis and centre are mechanically linked in a 'chirality relay' which is manifested in the complete inversion of the axial configuration on conversion into acyl derivatives such as 17 (ref. 8). The structural features of the lactam 15 and amine 16 were established by X-ray crystallography and computational methods.
For further analysis of the dibenz[c,e]azepine system we prepared the amines 18 (ref. 9), and observed that the C(5) and C(7) methyl groups in 18a and 18b favour a conformation in which these groups are pseudoequatorial. However, the C(4) methyl group in 18c tips the preference in favour of a pseudoaxial 5-methyl group, and with it the alternative (aR) configuration at the biaryl axis. The conformational bias in these amines is finely balanced: at room temperatures all of the structures are of the tropos (freely inverting) type.

Selected Publications
- Rapid Stereoselective Access to the Tetracyclic Core of Puupehenone and Related Sponge Metabolites Using Metal-free Radical Cyclisations of Cyclohexenyl-substituted 3-Bromochroman-4-ones.
R. G. Pritchard, H. M. Sheldrake, I. Z. Taylor and T. W. Wallace, Tetrahedron Lett., 2008, 49, 4156–4159.
DOI: https://doi.org/10.1016/j.tetlet.2008.04.114 - Conjugate Addition to 3-Arylsulfinylchromones as a Synthetic Route to Homochiral 2-Substituted Chromanones: Scope and Limitations.
K. J. Hodgetts, K. I. Maragkou, T. W. Wallace and R. C. R. Wootton, Tetrahedron, 2001, 57, 6793–6804.
DOI: https://doi.org/10.1016/S0040-4020(01)00615-9 - Tubulin-binding dibenz[c,e]oxepines: Part 2. Structural variation and biological evaluation as tumour vasculature disrupting agents.
S. B. Rossington, J. A. Hadfield, S. D. Shnyder, T. W. Wallace and K. J. Williams, Bioorg. Med. Chem., 2017, 25, 1630–1642.
DOI: https://doi.org/10.1016/j.bmc.2017.01.027 - Chemical Compounds with a Vascular Damaging Effect.
T. W. Wallace, D. J. Edwards and J. A. Hadfield, PCT Int. Appl. WO 2008075048 (26 June 2008).
Tubulin-binding dibenz[c,e]oxepines as colchinol analogues for targeting tumour vasculature.
D. J. Edwards, J. A. Hadfield, T. W. Wallace and S. Ducki, Org. Biomol. Chem., 2011, 9, 219–231.
DOI: https://doi.org/10.1039/C0OB00500B - Functionalised Cyclobutenes via Multicomponent Thermal [2 + 2] Cycloaddition Reactions.
H. M. Sheldrake, T. W. Wallace and C. P. Wilson, Org. Lett., 2005, 7, 4233–4236.
DOI: https://doi.org/10.1021/ol051675f - Biaryl Synthesis with Control of Axial Chirality.
T. W. Wallace, Org. Biomol. Chem., 2006, 4, 3197–3210.
DOI: https://doi.org/10.1039/B608470M - Kinetic and thermodynamic control of axial chirality in biaryl-derived fused oxazolidine lactams exploiting a centre-axis relay of unit efficiency.
D. J. Edwards, D. House, H. M. Sheldrake, S. J. Stone and T. W. Wallace, Org. Biomol. Chem., 2007, 5, 2658–2669.
DOI: https://doi.org/10.1039/B706336A - Enantioselective Route to 5-Methyl- and 5,7-Dimethyl-6,7-dihydro-5H-dibenz[c,e]azepine: Secondary Amines with Switchable Axial Chirality.
S. L. Pira, T. W. Wallace and J. P. Graham, Org. Lett., 2009, 11, 1663–1666.
DOI: https://doi.org/10.1021/ol900333c
Atroposelective formation of dibenz[c,e]azepines via intramolecular direct arylation with centre-axis chirality transfer.
C. A. Cheetham, R. S. Massey, S. L. Pira, R. G. Pritchard and T. W. Wallace, Org. Biomol. Chem., 2011, 9, 1831–1838.
DOI: https://doi.org/10.1039/C0OB00889C - Axial stereocontrol in tropos dibenz[c,e]azepines: the individual and cooperative effects of alkyl substituents.
S. M. C. Balgobin, D. J. Brookes, J. Jiang, R. G. Pritchard and T. W. Wallace, Org. Biomol. Chem., 2017, 15, 10184–10199.
DOI: https://doi.org/10.1039/C7OB02385E
